Spherical Binderless 4A/5A Zeolite Assemblies: Synthesis, Characterization, and Adsorbent Applications
Abstract
:1. Introduction
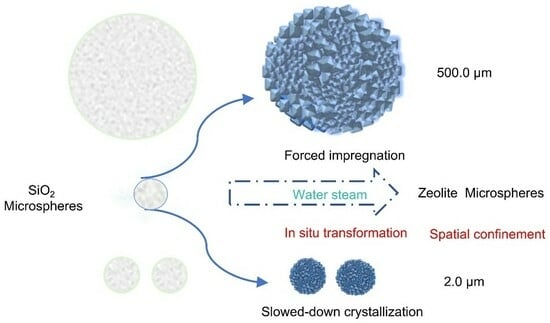
2. Results and Discussion
2.1. Synthesis of Downsized Sub-2-μm 4A Microspheres
2.2. Synthesis of Enlarged Sub-Millimeter 4A Spheres
2.3. Air Separation and Waste Removal Performance
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of Zeolite Microspheres
3.2.1. Synthesis of 2-Micron-Sized LTA Zeolite Microspheres
3.2.2. Synthesis of Millimeter-Sized LTA Zeolite
3.2.3. Calcium Cation Exchange
3.2.4. Ammonia Nitrogen Adsorption Experiments
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerstens, D.; Smeyers, B.; Van Waeyenberg, J.; Zhang, Q.; Yu, J.; Sels, B.F. State of the art and perspectives of hierarchical zeolites: Practical overview of synthesis methods and use in catalysis. Adv. Mater. 2020, 32, 2004690. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.R.; Lee, C.H.; Hamm, S.Y. Synthesis and characteristics of Na-A zeolite from natural kaolin in Korea. Mater. Chem. Phys. 2021, 261, 124230. [Google Scholar] [CrossRef]
- Rozhkovskaya, A.; Rajapakse, J.; Millar, G.J. Synthesis of high-quality zeolite LTA from alum sludge generated in drinking water treatment plants. J. Environ. Chem. Eng. 2021, 9, 104751. [Google Scholar] [CrossRef]
- Moreira, J.C.; Santa, R.A.A.B.; Nones, J.; Riella, H.G. Synthesis of zeolite 4a for obtaining zeolite 5A by ionic exchange for full utilization of waste from paper industry. Braz. J. Chem. Eng. 2018, 35, 623–630. [Google Scholar] [CrossRef]
- Grabias-Blicharz, E.; Panek, R.; Franus, M.; Franus, W. Mechanochemically assisted coal fly ash conversion into zeolite. Materials 2022, 15, 7174. [Google Scholar] [CrossRef]
- Panasenko, A.E.; Shichalin, O.O.; Yarusova, S.B.; Ivanets, A.I.; Belov, A.A.; Dran’kov, A.N.; Azon, S.A.; Fedorets, A.N.; Buravlev, I.Y.; Mayorov, V.Y.; et al. A novel approach for rice straw agricultural waste utilization: Synthesis of solid aluminosilicate matrices for cesium immobilization. Nucl. Eng. Technol. 2022, 54, 3250–3259. [Google Scholar] [CrossRef]
- Nikolopoulos, N.; Wickramasinghe, A.; Whiting, G.T.; Weckhuysen, B.M. Alumina binder effects on the hydrothermal stability of shaped zeolite-based catalyst bodies. Catal. Sci. Technol. 2022, 13, 862–873. [Google Scholar] [CrossRef]
- Whiting, G.T.; Chung, S.H.; Stosic, D.; Chowdhury, A.D.; Van Der Wal, L.I.; Fu, D.; Zecevic, J.; Travert, A.; Houben, K.; Baldus, M.; et al. Multiscale mechanistic insights of shaped catalyst body formulations and their impact on catalytic properties. ACS Catal. 2019, 9, 4792–4803. [Google Scholar] [CrossRef]
- Gökçal, B.; Kip, Ç.; Tuncel, A. Monodisperse-porous silica microspheres with flexible phenylboronic acid functionalized-polycationic molecular brushes as a sorbent for teamed boronate affinity chromatography in batch and capillary column systems. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132143. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in adsorption processes: State of the art and future prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef]
- Gao, P.; Fan, X.; Wang, W.; Yang, C. Sustainable technologies for adsorptive removal of estrogens from water: A comprehensive review for current advances. J. Environ. Chem. Eng. 2023, 11, 110780. [Google Scholar] [CrossRef]
- Lawson, S.; Newport, K.; Al-Naddaf, Q.; Ameh, A.E.; Rownaghi, A.A.; Petrik, L.F.; Rezaei, F. Binderless zeolite monoliths production with sacrificial biopolymers. Chem. Eng. J. 2021, 407, 128011. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, Z.; Fusi, N.; Liu, C.; Xiong, G.; Vilé, G.; He, N. Structured binder-free Al-β zeolite for acid-catalyzed dehydration. ACS Appl. Nano Mater. 2021, 4, 11997–12005. [Google Scholar] [CrossRef]
- Fawaz, E.G.; Salam, D.A.; Nouali, H.; Deroche, I.; Rigolet, S.; Lebeau, B.; Jean Daou, T. Synthesis of binderless ZK-4 zeolite microspheres at high temperature. Molecules 2018, 23, 2647. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, X.; Xiong, G.; Nie, B.; Liu, C.; He, N.; Liu, J. Towards the preparation of binderless ZSM-5 zeolite catalysts: The crucial role of silanol nests. Catal. Sci. Technol. 2020, 10, 7829–7841. [Google Scholar] [CrossRef]
- Boer, D.G.; Langerak, J.; Bakker, B.; Pescarmona, P.P. Binderless zeolite LTA beads with hierarchical porosity for selective CO2 adsorption in biogas upgrading. Microporous Mesoporous Mater. 2022, 344, 112208. [Google Scholar] [CrossRef]
- Miao, K.; Gao, J.; Zhang, J.; Dong, L.; Jia, Q.; Wang, R.; Yu, L.; Wang, S.; Hong, M.; Yang, S. Kinetics guided synthesis and performance of monodisperse zeolite LTA microspheres. Microporous Mesoporous Mater. 2021, 323, 111194. [Google Scholar] [CrossRef]
- Moukahhal, K.; Lebeau, B.; Josien, L.; Galarneau, A.; Toufaily, J.; Hamieh, T.; Daou, T.J. Synthesis of hierarchical zeolites with morphology control: Plain and hollow spherical beads of silicalite-1 nanosheets. Molecules 2020, 25, 2563. [Google Scholar] [CrossRef]
- Wang, Y.; Ai, F.; Ng, S.C.; Tan, T.T.Y. Sub-2 μm porous silica materials for enhanced separation performance in liquid chromatography. J. Chromatogr. A 2012, 1228, 99–109. [Google Scholar] [CrossRef]
- Fait, F.; Steinbach, J.C.; Kandelbauer, A.; Mayer, H.A. Incorporation of silica nanoparticles into porous templates to fabricate mesoporous silica microspheres for high performance liquid chromatography applications. J. Chromatogr. A 2023, 1705, 464190. [Google Scholar] [CrossRef]
- Weissenberger, T.; Reiprich, B.; Machoke, A.G.F.; Klühspies, K.; Bauer, J.; Dotzel, R.; Casci, J.L.; Schwieger, W. Hierarchical MFI type zeolites with intracrystalline macropores: The effect of the macropore size on the deactivation behaviour in the MTO reaction. Catal. Sci. Technol. 2019, 9, 3259–3269. [Google Scholar] [CrossRef]
- Awala, H.; Gilson, J.P.; Retoux, R.; Boullay, P.; Goupil, J.M.; Valtchev, V.; Mintova, S. Template-free nanosized faujasite-type zeolites. Nat. Chem. 2015, 14, 447–451. [Google Scholar] [CrossRef]
- Yu, S.; Kwon, S.; Na, K. Synthesis of LTA zeolites with controlled crystal sizes by variation of synthetic parameters: Effect of Na+ concentration, aging time, and hydrothermal conditions. J. Sol.-Gel Sci. Technol. 2021, 98, 411–421. [Google Scholar] [CrossRef]
- Chen, H.; Wydra, J.; Zhang, X.; Lee, P.S.; Wang, Z.; Fan, W.; Tsapatsis, M. Hydrothermal synthesis of zeolites with three-dimensionally ordered mesoporous-imprinted structure. J. Am. Chem. Soc. 2011, 133, 12390–12393. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef]
- Chen, C.; Zhai, D.; Dong, L.; Wang, Y.; Zhang, J.; Liu, Y.; Chen, Z.; Qian, W.; Hong, M. Organic anions facilitate in situ synthesis of mesoporous LTA zeolites. Chem. Mater. 2019, 31, 1528–1536. [Google Scholar] [CrossRef]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as Selective Adsorbents for CO2 Separation. ACS Appl. Energy Mater. 2023, 6, 2634–2656. [Google Scholar] [CrossRef]
- Ackley, M.W. Medical oxygen concentrators: A review of progress in air separation technology. Adsorption 2019, 25, 1437–1474. [Google Scholar] [CrossRef]
- Rao, B.; Dai, H.; Gao, L.; Xie, H.; Gao, G.; Peng, K.; Zhang, M.; He, F.; Pan, Y. Surprisingly highly reactive silica that dissolves rapidly in dilute alkali (NaOH) solution even at ambient temperatures (25 °C). J. Clean. Prod. 2022, 341, 130779. [Google Scholar] [CrossRef]
- Muraoka, K.; Sada, Y.; Miyazaki, D.; Chaikittisilp, W.; Okubo, T. Linking synthesis and structure descriptors from a large collection of synthetic records of zeolite materials. Nat. Commun. 2019, 10, 4459. [Google Scholar] [CrossRef]
- Oleksiak, M.D.; Soltis, J.A.; Conato, M.T.; Penn, R.L.; Rimer, J.D. Nucleation of FAU and LTA zeolites from heterogeneous aluminosilicate precursors. Chem. Mater. 2016, 28, 4906–4916. [Google Scholar] [CrossRef]
- Manickam, S.; Boffito, D.C.; Flores, E.M.M.; Leveque, J.M.; Pflieger, R.; Pollet, B.G.; Ashokkumar, M. Ultrasonics and sonochemistry: Editors’ perspective. Ultrason. Sonochemistry 2023, 99, 106540. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, N.; Chen, Y.; Zhao, J.; Zhu, R.; Hong, M. Recent advances in synthetic strategies and physicochemical modifications of SSZ-13 zeolites: A review. Mater. Today Catal. 2024, 4, 100037. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Papynov, E.K.; Ivanov, N.P.; Balanov, M.I.; Dran’kov, A.N.; Shkuratov, A.L.; Zarubina, N.V.; Fedorets, A.N.; Mayorov, V.Y.; Lembikov, A.O.; et al. Study of adsorption and immobilization of Cs+, Sr2+, Co2+, Pb2+, La3+ ions on Na-Faujasite zeolite transformed in solid state matrices. Sep. Purif. Technol. 2024, 332, 125662. [Google Scholar] [CrossRef]
- Azhagapillai, P.; Khaleel, M.; Zoghieb, F.; Luckachan, G.; Jacob, L.; Reinalda, D. Water vapor adsorption capacity loss of molecular sieves 4A, 5A, and 13X resulting from methanol and heptane exposure. ACS Omega 2022, 7, 6463–6471. [Google Scholar] [CrossRef]
- Wang, J.; Huo, X.; Zhang, F.; Wang, L.; Wang, X.; Li, J.; Yang, J. Separation of cobalt and lithium from spent LiCoO2 batteries using zeolite NaA and the resulting ion exchange product for N2/O2 separation. Sep. Purif. Technol. 2023, 313, 123449. [Google Scholar] [CrossRef]
- Kim, J.; Jung, T.; Cho, D.W.; Yoo, C.Y. Comprehensive evaluation of 3A, 4A, 5A, and 13X zeolites for selective 1-octene adsorption over n-octane. J. Ind. Eng. Chem. 2022, 110, 274–285. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Yu, B.; Zheng, G.; Zhao, J.; Hong, M. Amino acid mediated mesopore formation in LTA zeolites. J. Mater. Chem. A. 2016, 4, 2305–2313. [Google Scholar] [CrossRef]
- Mofarahi, M.; Seyyedi, M. Pure and binary adsorption isotherms of nitrogen and oxygen on zeolite 5A. J. Chem. Eng. Data 2009, 54, 916–921. [Google Scholar] [CrossRef]
- Edwards, T.M.; Puglis, H.J.; Kent, D.B.; Durán, J.L.; Bradshaw, L.M.; Farag, A.M. Ammonia and aquatic ecosystems—A review of global sources, biogeochemical cycling, and effects on fish. Sci. Total Environ. 2024, 907, 167911. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, Q.; Xing, Z. Adsorption of ammonia nitrogen in low temperature domestic wastewater by modification bentonite. J. Clean. Prod. 2019, 233, 720–730. [Google Scholar] [CrossRef]










| a\AA a | Si/Al b | Si/Al c | Vmicro d (cm3/g) | Vtotal e (cm3/g) | SBET f (m2/g) | |
|---|---|---|---|---|---|---|
| Si-2 | - | - | - | - | 0.93 | 495 |
| Si-500 | - | - | - | - | 0.21 | 263 |
| NaA-2 | 25.020 | 1.01 | 1.00 | 0.19 | 0.97 g | 501 g |
| NaA-500 | 24.996 | 1.07 | 1.00 | 0.23 | 0.54 g | 357 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Wang, S.; Gao, J.; Wang, R.; Gao, G.; Ren, G.; Na, S.; Hong, M.; Yang, S. Spherical Binderless 4A/5A Zeolite Assemblies: Synthesis, Characterization, and Adsorbent Applications. Molecules 2024, 29, 1432. https://doi.org/10.3390/molecules29071432
Li T, Wang S, Gao J, Wang R, Gao G, Ren G, Na S, Hong M, Yang S. Spherical Binderless 4A/5A Zeolite Assemblies: Synthesis, Characterization, and Adsorbent Applications. Molecules. 2024; 29(7):1432. https://doi.org/10.3390/molecules29071432
Chicago/Turabian StyleLi, Tong, Shuangwei Wang, Jinqiang Gao, Ruiqiang Wang, Guifeng Gao, Guangming Ren, Shengnan Na, Mei Hong, and Shihe Yang. 2024. "Spherical Binderless 4A/5A Zeolite Assemblies: Synthesis, Characterization, and Adsorbent Applications" Molecules 29, no. 7: 1432. https://doi.org/10.3390/molecules29071432
APA StyleLi, T., Wang, S., Gao, J., Wang, R., Gao, G., Ren, G., Na, S., Hong, M., & Yang, S. (2024). Spherical Binderless 4A/5A Zeolite Assemblies: Synthesis, Characterization, and Adsorbent Applications. Molecules, 29(7), 1432. https://doi.org/10.3390/molecules29071432