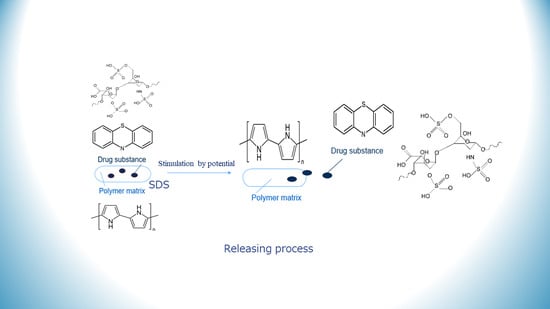
Chlorpromazine–Polypyrrole Drug Delivery System Tailored for Neurological Application
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of CPZ/HEP/PPy in Sodium Dodecyl Sulfate Solution
2.2. Electrochemical Properties of Polymers
2.3. Structural Analysis of PPy-Derived Films
2.4. Microstructural and Surface Properties of PPy-Derived Films
2.5. Release Studies
3. Materials and Methods
3.1. Materials
3.2. Polymerization of PPy-Derived Films via Cyclic Voltammetry and Chronoamperometry Procedures Used to Stimulate the Release of Entrapped CPZ and HEP
3.3. Characterization of CPZ/HEP/PPy Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Grabowska, M.E.; Huang, A.; Wen, Z.; Wei, W.Q. Drug repurposing for Alzheimer’s disease from 2012–2022—A 10-year literature review. Front. Pharmacol. 2023, 14, 1257700. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Rahfeld, J.-U.; Lues, I.; Lemere, C. Passive Aβ Immunotherapy: Current Achievements and Future Perspectives. Molecules 2018, 23, 1068. [Google Scholar] [CrossRef]
- Sonawane, S.; Bhanvase, B.A.; Sivakumar, M. Encapsulation of Active Molecules and Their Delivery System; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Rouabhia, M.; Moulton, S.E. Conductive Polymers: Electrical Interactions in Cell Biology and Medicine, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Distler, T.; Polley, C.; Shi, F.; Schneidereit, D.; Ashton, M.D.; Friedrich, O.; Boccaccini, A.R. Electrically Conductive and 3D-Printable Oxidized Alginate-Gelatin Polypyrrole: PSS Hydrogels for Tissue Engineering. Adv. Healthc. Mater. 2010, 10, 2001876. [Google Scholar] [CrossRef] [PubMed]
- El Mhammedi, M.; Kinani, A.L.; Chtaini, A. Synthesis and Polymerization of Pyrole Characterization of Polypyrole. Leonardo Electron. J. Pract. Technol. 2007, 46, 1–6. [Google Scholar]
- Shah, S.A.A.; Firlak, M.; Berrow, S.R.; Halcovitch, N.S.; Baldock, S.J.; Yousafzai, B.M.; Hathout, R.M.; Hardy, J.G. Electrochemically Enhanced Drug Delivery Using Polypyrrole Films. Materials 2018, 11, 1123. [Google Scholar] [CrossRef] [PubMed]
- Tüken, T. Polypyrrole films on stainless steel. Surf. Coat. Technol. 2006, 200, 4713–4719. [Google Scholar] [CrossRef]
- Gerard, M.; Chaubey, A.; Malhotra, B.D. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Shamaeli, E.; Alizadeh, N. Nanostructured biocompatible thermal/electrical stimuli-responsive biopolymer-doped polypyrrole for controlled release of chlorpromazine: Kinetics studies. Int. J. Pharm. 2014, 472, 327–338. [Google Scholar] [CrossRef]
- Gomez, N.; Schmid, C.E. Nerve growth factor-immobilized polypyrrole: Bioactiveelectrically conducting polymer for enhancedneurite extension. J. Biomed. Mater. Res. Part A 2007, 81, 135–149. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Lyckman, A.W.; LaVan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Broeders, J.J.; Hermens, J.L.; Blaauboer, B.J.; Zurich, M.-G. The in vitro biokinetics of chlorpromazine and diazepam in aggregating rat brain cell cultures after repeated exposure. Toxicol. In Vitro 2015, 30, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, Y.; Ijima, H. Development of heparin-conjugated nanofibers and a novel biological signal by immobilized growth factors for peripheral nerve regeneration. J. Biosci. Bioeng. 2019, 129, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Durgam, H.; Sapp, S.; Deister, C.; Khaing, Z.; Chang, E.; Luebben, S.; Schmidt, C.E. Novel Degradable Co-polymers of Polypyrrole Support Cell Proliferation and Enhance Neurite Out-Growth with Electrical Stimulation. J. Biomater. Sci. Polym. Ed. 2010, 21, 1265–1282. [Google Scholar] [CrossRef]
- Yi, N.; Abidian, M.R. Conducting Polymers and Their Biomedical Applications, Biosynthetic Polymers for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2016; pp. 243–270. [Google Scholar]
- Fahlgren, A.; Bratengeier, C.; Gelmi, A.; Semeins, C.M.; Klein-Nulend, J.; Jager, E.W.H.; Bakker, A.D. Biocompatibility of Polypyrrole with Human Primary Osteoblasts and the Effect of Dopants. PLoS ONE 2015, 10, e0134023. [Google Scholar] [CrossRef] [PubMed]
- Hepel, J.; Bruckenstein, S.; Hepel, M. Effect of pH on Ion Dynamics in Composite PPy/Heparin Films. Microchem. J. 1997, 55, 179–191. [Google Scholar] [CrossRef]
- Flamini, D.O.; González, M.B.; Saidman, S.B. Synthesis and Characterization of Heparin-Doped Polypyrrole Coatings Using an Electrochemical Quartz Crystal Microbalance (EQCM). Port. Electrochim. Acta 2022, 40, 47–57. [Google Scholar] [CrossRef]
- Liechty, W.B.; Scheuerle, R.L.; Vela Ramirez, J.E.; Peppas, N.A. Uptake and function of membrane-destabilizing cationic nanogels for intracellular drug delivery. Bioeng. Transl. Med. 2019, 4, 17–29. [Google Scholar] [CrossRef]
- Baloch, J.; Farhan Sohail, M.; Shaib, H.S.; Kiani, M.H.; Majid Khan, G.; Jahan, S.; Rafay, M.; Tausif Chaudhry, M.; Yasinzai, M.; Shahnaz, G. Self-Nanoemulsifying Drug Delivery System (SNEDDS) for Improved Oral Bioavailability of Chlorpromazine: In Vitro and In Vivo Evaluation. Medicina 2019, 55, 210. [Google Scholar] [CrossRef] [PubMed]
- Hussain, W.; Kushwaha, P.; Rahman, A.; Akhtar, J. Development and Evaluation of Fast Dissolving Film for Oro-Buccal Drug Delivery of Chlorpromazine. Indian J. Pharm. Educ. Res. 2017, 51, s539–s547. [Google Scholar] [CrossRef]
- Garner, B.; Georgevich, A.; Hodgson, A.J.; Liu, L.; Wallace, G.G. Polypyrrole–Heparin Composites as Stimulus-Responsive Substrates for Endothelial Cell Growth; John Wiley and Sons Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Ratautaite, V.; Boguzaite, R.; Beatrice Mickeviciute, M.; Mikoliunaite, L.; Samukaite-Bubniene, U.; Ramanavicius, A.; Ramanaviciene, R. Evaluation of Electrochromic Properties of Polypyrrole/Poly(Methylene Blue) Layer Doped by Polysaccharides. Sensors 2022, 22, 232. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Zhang, A.; Shao, X.; Liu, T.; Tang, B.; Fang, G. Strategies for sustained release of heparin: A review. Carbohydr. Polym. 2022, 294, 119793. [Google Scholar] [CrossRef] [PubMed]
- Del Pilar Rodriguez-Torres, M.; Díaz-Torres, L.A.; Romero-Servin, S. Heparin Assisted Photochemical Synthesis of Gold Nanoparticles and Their Performance as SERS Substrates. Int. J. Mol. Sci. 2014, 15, 19239–19252. [Google Scholar] [CrossRef] [PubMed]
- Golkhatmi, S.Z.; Sedghi, A.; Miankushki, H.N.; Khalaj, M. Structural Properties and Supercapacitive Performance Evaluation of the Nickel Oxide/Graphene/Polypyrrole Hybrid Ternary Nanocomposite in Aqueous and Organic Electrolytes. Energy 2020, 214, 118950. [Google Scholar] [CrossRef]
- Schweiger, B.; Kim, J.; Kim, Y.; Ulbricht, M. Electropolymerized Molecularly Imprinted Polypyrrole Film for Sensing of Clofibric Acid. Sensors 2015, 15, 4870. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lee, T.; Paik, W. Electroactive counter anions in conducting polypyrrole: Hexacyanoferrate and heteropolytungstate ions. Synth. Met. 1995, 69, 485–486. [Google Scholar] [CrossRef]
- Wang, Q.; Deng, Y.; Chen, J.; Lu, L.; Ma, Y.; Zang, L. Electrochemical preparation of polypyrrole-Ag nanoparticles composite film and its resistive switching properties. J. Alloys Compd. 2022, 927, 167117, ISSN 0925-8388. [Google Scholar] [CrossRef]
- Holze, R. Overoxidation of Intrinsically Conducting Polymers. Polymers 2022, 14, 1584. [Google Scholar] [CrossRef] [PubMed]
- Samwang, T.; Watanabe, N.M.; Okamoto, Y.; Srinives, S.; Umakoshi, H. Study of Chemical Polymerization of Polypyrrole with SDS Soft Template: Physical, Chemical, and Electrical Properties. ACS Omega 2023, 8, 48946–48957. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jang, Y.J.; Kim, D.W.; Cheruku, R.; Thogiti, S.; Ahn, K.-S.; Kim, J.H. Application of polypyrrole/sodium dodecyl sulfate/carbon nanotube counter electrode for solid-state dye-sensitized solar cells and dye-sensitized solar cells. Chem. Pap. 2019, 73, 2749–2755. [Google Scholar] [CrossRef]
- Skopek, M.A.; Mohamoud, M.A.; Ryder, K.S.; Hillman, A.R. Nanogravimetric observation of unexpected ion exchange characteristics for polypyrrole film p-doping in a deep eutectic ionic liquid. Chem. Commun. 2009, 131, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Cascales, L.; Otero, T.F. Molecular dynamic simulation of the hydration and diffusion of chloride ions from bulk water to polypyrrole matrix. J. Chem. Phys. 2004, 120, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Annibaldi, V.; Rooney, A.D.; Breslin, C.B. Corrosion protection of copper using polypyrrole electrosynthesised from a salicylate solution. Corros. Sci. 2012, 59, 179–185. [Google Scholar] [CrossRef]
- Hallik, A.; Roosalu, K.; Mändar, H.; Joosu, L.; Marandi, M.; Tamm, J. Thickness dependence of the porosity of PPy/DDS films. Eur. Polym. J. 2015, 70, 118–124. [Google Scholar] [CrossRef]
- Ryan, E.M.; Breslin, C.B. Formation of polypyrrole with dexamethasone as a dopant: Its cation and anion exchange properties. J. Electroanal. Chem. 2018, 824, 188–194. [Google Scholar] [CrossRef]
- Arroyoa, J.; Akieh-Pirkanniemia, M.; Lisakb, G.; Latonena, R.M.; Bobacka, J. Electrochemically controlled transport of anions across polypyrrole-based membranes. J. Membr. Sci. 2019, 581, 50–57. [Google Scholar] [CrossRef]
- Bayat, M.; Izadan, H.; Molina, B.G.; Sánchez, M.; Santiago, S.; Semnani, D.; Dinari, M.; Guirado, G.; Estrany, F.; Alemán, C. Electrochromic Self-Electrostabilized Polypyrrole Films Doped with Surfactant and Azo Dye. Polymers 2019, 11, 1757. [Google Scholar] [CrossRef] [PubMed]
- Omastová, M.; Trchová, M.; Kovářová, J.; Stejskal, J. Synthesis and structural study of polypyrroles prepared in the presence of surfactants. Synthetic Metals 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Djebbi, M.A.; Boubakri, S.; Bouaziz, Z.; Elayachi, M.S.; Namour, P.; Jaffrezic-Renault, N.; Amara, A.B.H. Extended-release of chlorpromazine intercalated into montmorillonite clays. Microporous Mesoporous Mater. 2018, 267, 43–52, ISSN 1387-1811. [Google Scholar] [CrossRef]
- Grant, D.; Long, W.F.; Moffat, C.F.; Williamson, F.B. Infrared spectroscopy of heparins suggests that the region 750-950 cm is sensitive to changes in iduronate residue ring conformation. Biochem. J. 1991, 275, 193–197. [Google Scholar] [CrossRef]
- Liang, W.; Lei, J.; Martin, C.R. Effect of synthesis temperature on the structure, doping level and charge-transport properties of polypyrrole. Synth. Met. 1992, 52, 227–239. [Google Scholar] [CrossRef]
- Frère, P.; Raimundo, J.M.; Blanchard, P.; Delaunay, J.; Richomme, P.; Sauvajol, J.L.; Orduna, J.; Garin, J.; Roncali, J. Effect of Local Molecular Structure on the Chain-Length Dependence of the Electronic Properties of Thiophene-Based π-conjugated systems. J. Org. Chem. 2003, 68, 7254–7265. [Google Scholar] [CrossRef] [PubMed]
- Namsheer, K.; Sekhar Rout, C. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Gandhi, M.R. Morphological Studies and Structure-Property Relationship of Polypyrrole. Master’s Thesis, Department of Materials Engineering, University of Wollongong, Wollongong, NSW, Australian, 1993. Available online: https://ro.uow.edu.au/theses/2495 (accessed on 25 February 2024).
- Asplund, A.; Nyberg, T.; Inganäs, O. Electroactive polymers for neural interfaces. Polym. Chem. 2010, 1, 1374–1391. [Google Scholar] [CrossRef]
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting polymers, hydrogels and their composites: Preparation, properties and bioapplications. Polymers 2019, 11, 350. [Google Scholar] [CrossRef]
- Hazarika, J.; Kumar, A. Controllable synthesis and characterization of polypyrrole nanoparticles in sodium dodecylsulphate (sSDS) micellar solution. Synth. Met. 2013, 175, 155–162. [Google Scholar] [CrossRef]
- Maw, A.M.; Theingi, M.; Than Yee, K. Polypyrrole-sodium bentonite composites and its electrical and optical properties. J. Myanmar Acad. Arts Sci. 2020, XVIII, 797–806. [Google Scholar]
- Chougule, M.A.; Pawar, S.G.; Godse, P.R.; Mulik, R.N.; Sen, S.; Patil, V.B. Synthesis and Characterization of Polypyrrole (PPy) Thin Films. Soft Nanosci. Lett. 2011, 1, 6–10. [Google Scholar] [CrossRef]
- Waghuley, S.A.; Yenorkar, S.M.; Yawale, S.S.; Yawale, S.P. Application of chemically synthesized conducting polymer-polypyrrole as a carbon dioxide gas sensor. Sens. Actuators B Chem. 2008, 128, 366–373. [Google Scholar] [CrossRef]
- Suwalsky, M.; Gimenez, L.; Saenger, V.; Neira, F. X-Ray Studies on Phospholipid Bilayers. VIII. Interactions with Chlorpromazine · HCl. Z. Für Naturforschung C 1998, 43, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Sanches, E.A.; Alves, S.F.; Soares, J.C.; da Silva, A.M.; da Silva, C.G.; de Souza, S.M.; da Frota, H.O. Nanostructured Polypyrrole Powder: A Structural and Morphological Characterization. J. Nanomater. 2015, 2015, 129678. [Google Scholar] [CrossRef]
- Lan James, B. Adsorption and Aggregation of Sodium Dodecyl Sulfate on au(iii) Electrode Surfaces. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2000. [Google Scholar]
- Hou, M.; Jiang, Z.; Chu, F.; Zhang, X.; Lai, N.-C. Kinetics of micelle formation and their effect on the optical and structural properties of polypyrrole nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 385, 55–62. [Google Scholar] [CrossRef]
- Bikshapathia, M.; Singha, S.; Bhaduria, B.; Mathura, G.N.; Sharmaa, A.; Vermaa, N. Surfactant-mediated preparation and application to the removal of gaseous VOCs. Colloids Surf. A Physicochem. Eng. Asp. 2012, 399, 46–55. [Google Scholar] [CrossRef]
- Panero, S.; Moreno, J.S.; Aleandri, P.; Landi, E.; Sprio, S.; Tampieri, A. Porous Hydroxyapatite Surface-Modified by Polypyrrole-Heparin Conducting Polymer. Key Eng. Mater. 2007, 361–363, 443–446. [Google Scholar] [CrossRef]
- Jiang, S.; Qiao, C.; Wang, X.; Li, Z.; Yang, G. Structure and properties of chitosan/ sodium dodecyl sulfate composites films. RSC Adv. 2022, 12, 3969–3978. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Qin, J.; Zhang, X.; Li, R.; Thueploy, A.; Limpanart, S.; Liu, R. Effect of Sodium Dodecyl Sulphate and Sodium Bromide Additives on Ni–W Nanocoatings. J. Nanosci. Nanotechnol. 2017, 17, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, X.; Maclean, M.; Qin, Y.; Duxbury, M.; Ding, F. A single-step fabrication approach for development of antimicrobial surfaces. J. Mater. Process. Technol. 2019, 271, 249–260. [Google Scholar] [CrossRef]
- Syugaev, A.V.; Lyalina, N.V.; Maratkanova, A.N.; Smirnov, D.A. Molecular architecture of highly protective coatings of electrodeposited dodecyl sulfate-doped polypyrrole. Prog. Org. Coat. 2019, 131, 427–434. [Google Scholar] [CrossRef]
- Smela, E.; Gadegaard, N. Volume Change in Polypyrrole Studied by Atomic Force Microscopy. J. Phys. Chem. B 2001, 105, 9395–9405. [Google Scholar] [CrossRef]
- Weize Yuan, W.; Harikrishnan Vijayamohanan, H.; Shao-Xiong, L.L.; Husted, K.; Johnson, A.J.; Swag, T.M. Dynamic Polypyrrole Core–Shell Chemomechanical Actuators. Chem. Mater. 2022, 34, 3013–3019. [Google Scholar] [CrossRef]
- Sirivisoot, S.; Pareta, R.; Webster, T.J. Electrically controlled drug release from nanostructured polypyrrole coated on titanium. Nanotechnology 2011, 22, 085101–085116. [Google Scholar] [CrossRef]
- Zhang, B.; Molino, P.J.; Harris, A.R.; Yue, Z.; Moulton, S.E.; Wallace, G.G. Conductive and protein resistant polypyrrole films for dexamethasone delivery. J. Mater. Chem. B 2014, 4, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, M.; Ma, H.R. Morphology of Polypyrrole Prepared in SDS Solution. Adv. Mater. Res. 2012, 554–556, 303–306. [Google Scholar] [CrossRef]
- Fujikawa, K.; Jung, H.S.; Park, J.W.; Kim, J.M.; Lee, H.Y.; Kawai, T. AFM imaging of nanostructure polypyrrole doughnuts shapes fabricated by direct electrochemical oxidation. Electrochem. Commun. 2004, 6, 461–464. [Google Scholar] [CrossRef]
- González-Torres, M.; Olayo, M.G.; Gómez, L.M. Chemical interactions of heparin in porous polypyrrole, an example of drug–carrier destructive interaction. Polym. Bull. 2020, 77, 375–385. [Google Scholar] [CrossRef]
- Svirskis, D.; Sharma, M.; Yu, Y.; Garg, S. Electrically switchable polypyrrole film for the tunable release of progesterone. Ther. Deliv. 2013, 4, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Hallik, A.; Alumaa, A.; Kurig, H.; Jänes, A.; Lust, E.; Tamm, J. On the porosity of polypyrrole films. Synth. Met. 2007, 157, 1085–1090. [Google Scholar] [CrossRef]
- Sravanthi, M.; Manjunatha, K.G. Synthesis and characterization of conducting polypyrrole with various dopants. Mater. Today Proc. 2021, 46, 5964–5968. [Google Scholar] [CrossRef]
- Dudley, K.; Liu, X.; De Haan, S. Chlorpromazine dose for people with schizophrenia. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Wołowicz, A.; Staszak, K. Study of surface properties of aqueous solutions of sodium dodecyl sulfate in the presence of hydrochloric acid and heavy metal ions. J. Mol. Liq. 2019, 229, 112170. [Google Scholar] [CrossRef]
- Zhang, M.; Bao, W.-Q.; Wang, Y.; Deng, N.; He, J.-B. In situ monitoring of chlorpromazine radical intermediate by spectroelectrochemistry. J. Electroanal. Chem. 2014, 724, 1–7. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]






| Synthesis Parameters | Name | ES [%] | g [µm] |
|---|---|---|---|
| E1 = 0.85 V, cycles 20 | f1 | 92.50 | 7.80 |
| E1 = 0.85 V, cycles 30 | f2 | 105.64 | 13.50 |
| E2 = 0.70 V, cycles 20 | f3 | 94.080 | 3.52 |
| E2 = 0.70 V, cycles 30 | f4 | 110.52 | 5.20 |
| Film | Ra before Release [nm] | Ra after Release [nm] |
|---|---|---|
| f1 | 171.12 | 137.00 |
| f2 | 70.52 | 153.80 |
| f3 | 158.23 | 146.030 |
| f4 | 50.21 | 89.24 |
| Synthesis Parameters | Film | CPZ RE [%] | HEP RE [%] | EE CPZ [%] | m CPZ Released [mg/cm2] |
|---|---|---|---|---|---|
| E1 = 0.85 V, c. 20 | f1 | 75 0.18 | 77 0.13 | 0.64 0.020 | 15.26 0.12 |
| E1 = 0.85 V, c. 30 | f2 | 56 0.090 | 9.5 0.020 | 2.01 0.050 | 20.96 0.20 |
| E2 = 0.70 V, c. 20 | f3 | 82 0.23 | 11 0.080 | 0.070 | 17.79 0.18 |
| E2 = 0.70 V, c. 30 | f4 | 68 0.12 | 17 0.090 | 0.010 | 11.30 0.080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk, S.; Golba, S.; Neves, C.; Tedim, J. Chlorpromazine–Polypyrrole Drug Delivery System Tailored for Neurological Application. Molecules 2024, 29, 1531. https://doi.org/10.3390/molecules29071531
Krawczyk S, Golba S, Neves C, Tedim J. Chlorpromazine–Polypyrrole Drug Delivery System Tailored for Neurological Application. Molecules. 2024; 29(7):1531. https://doi.org/10.3390/molecules29071531
Chicago/Turabian StyleKrawczyk, Sara, Sylwia Golba, Cristina Neves, and João Tedim. 2024. "Chlorpromazine–Polypyrrole Drug Delivery System Tailored for Neurological Application" Molecules 29, no. 7: 1531. https://doi.org/10.3390/molecules29071531
APA StyleKrawczyk, S., Golba, S., Neves, C., & Tedim, J. (2024). Chlorpromazine–Polypyrrole Drug Delivery System Tailored for Neurological Application. Molecules, 29(7), 1531. https://doi.org/10.3390/molecules29071531