TDDFT Study on the ESIPT Properties of 2-(2′-Hydroxyphenyl)-Benzothiazole and Sensing Mechanism of a Derived Fluorescent Probe for Fluoride Ion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Geometric Configuration
2.2. Energy Analysis
2.3. Electron Spectrum
2.4. Excited State Proton Transfer
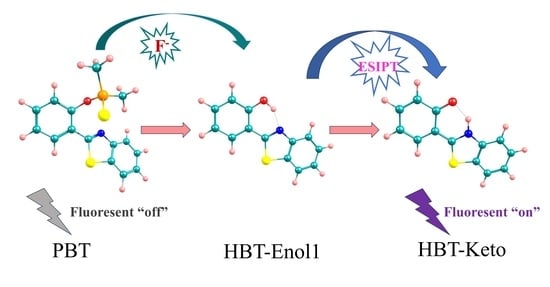
2.5. Sensing Mechanism
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molina, P.; Zapata, F.; Caballero, A. Anion Recognition Strategies Based on Combined Noncovalent Interactions. Chem. Rev. 2017, 117, 9907–9972. [Google Scholar] [CrossRef]
- Martínez-Máñez, R.; Sancenón, F. Fluorogenic and Chromogenic Chemosensors and Reagents for Anions. Chem. Rev. 2003, 103, 4419–4476. [Google Scholar] [CrossRef]
- Basri, R.; Ahmed, N.; Khalid, M.; Khan, M.U.; Abdullah, M.; Syed, A.; Elgorban, A.M.; Al-Rejaie, S.S.; Braga, A.A.C.; Shafiq, Z. Quinoline based thiosemicarbazones as colorimetric chemosensors for fluoride and cyanide ions and DFT studies. Sci. Rep. 2022, 12, 4927. [Google Scholar] [CrossRef]
- Zohoori, F.V.; Maguire, A. Development of a Database of the Fluoride Content of Selected Drinks and Foods in the UK. Caries Res. 2016, 50, 331–336. [Google Scholar] [CrossRef]
- Baruah, U.; Gogoi, N.; Majumdar, G.; Chowdhury, D. β-Cyclodextrin and calix[4]arene-25,26,27,28-tetrol capped carbon dots for selective and sensitive detection of fluoride. Carbohydr. Polym. 2015, 117, 377–383. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Maji, R.; Maiti, K.; Adhikari, S.S.; Mukhopadhyay, C.D.; Mandal, D. Ratiometric sensing of fluoride and acetate anions based on a BODIPY-azaindole platform and its application to living cell imaging. Analyst 2014, 139, 309–317. [Google Scholar] [CrossRef]
- Crystal, Y.O.; Marghalani, A.A.; Ureles, S.D.; Wright, J.T.; Sulyanto, R.; Divaris, K.; Fontana, M.; Graham, L. Use of silver diamine fluoride for dental caries management in children and adolescents, including those with special health care needs. Pediatr. Dent. 2017, 39, 135E–145E. [Google Scholar] [CrossRef]
- Jagtap, S.; Yenkie, M.K.; Labhsetwar, N.; Rayalu, S. Fluoride in Drinking Water and Defluoridation of Water. Chem. Rev. 2012, 112, 2454–2466. [Google Scholar] [CrossRef]
- Ding, S.; Xu, A.; Li, M.; Sun, A.; Zhang, Z.; Xia, Y.; Liu, Y. Theoretical study on the sensing mechanism of an ON1-OFF-ON2 type fluoride fluorescent chemosensor. Spectrochim. Acta Part A 2020, 237, 118397. [Google Scholar] [CrossRef]
- Jiao, S.; Wang, X.; Sun, Y.; Zhang, L.; Sun, W.; Sun, Y.; Wang, X.; Ma, P.; Song, D. A novel fluorescein-coumarin-based fluorescent probe for fluoride ions and its applications in imaging of living cells and zebrafish in vivo. Sens. Actuators B Chem. 2018, 262, 188–194. [Google Scholar] [CrossRef]
- Sivamani, J.; Siva, A. Self-assembly, “turn-on” fluorescent detection of fluoride ion using uracil based azo derivatives and their application in imaging of living cells. Sens. Actuators B Chem. 2017, 242, 423–433. [Google Scholar] [CrossRef]
- Liu, J.-M.; Lin, L.-P.; Wang, X.-X.; Jiao, L.; Cui, M.-L.; Jiang, S.-L.; Cai, W.-L.; Zhang, L.-H.; Zheng, Z.-Y. Zr(H2O)2EDTA modulated luminescent carbon dots as fluorescent probes for fluoride detection. Analyst 2013, 138, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.; Zhu, H.; Zhang, H.; Zhou, C.; Miao, T. Study on Kinetics of GB and GD hydrolysis by Peroxides with a way of fluoride ion-selective electrode. J. Phys. Conf. Ser. 2022, 2321, 012021. [Google Scholar] [CrossRef]
- Breadmore, M.C.; Palmer, A.S.; Curran, M.; Macka, M.; Avdalovic, N.; Haddad, P.R. On-Column Ion-Exchange Preconcentration of Inorganic Anions in Open Tubular Capillary Electrochromatography with Elution Using Transient-Isotachophoretic Gradients. 3. Implementation and Method Development. Anal. Chem. 2002, 74, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Jha, S.; Bhattacharya, S. Colorimetric Probes Based on Anthraimidazolediones for Selective Sensing of Fluoride and Cyanide Ion via Intramolecular Charge Transfer. J. Org. Chem. 2011, 76, 8215–8222. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Pei, X.; Li, C.; Zeng, W.; Chi, Y.; Chen, X.; Chai, S.; Chen, J. A dual colorimetric fluorescent probe with large Stokes shift for F- detection in the near infrared and its application in cell imaging. Inorg. Chim. Acta 2023, 555, 121589. [Google Scholar] [CrossRef]
- Shi, H.; Chen, H.; Li, X.; Xing, J.; Zhang, G.; Zhang, R.; Liu, J. A simple colorimetric and ratiometric fluoride ion probe with large color change. RSC Adv. 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Jiang, C.; Ye, X.; Wu, N.; Li, P.; Yang, L.; Liu, Y.; Fu, Y.; Ye, F. Development and application of fluorescent probes for the selective and sensitive detection of F- and oxyfluorfen. Inorg. Chim. Acta. 2021, 522, 120362. [Google Scholar] [CrossRef]
- Ma, X.; Hao, Y.; Liu, J.; Wu, G.; Liu, L. A Green-emitting Fluorescent Probe Based on a Benzothiazole Derivative for Imaging Biothiols in Living Cells. Molecules 2019, 24, 411. [Google Scholar] [CrossRef] [PubMed]
- Mohanasundaram, D.; Kumar, G.G.V.; Kumar, S.K.; Maddiboyina, B.; Raja, R.P.; Rajesh, J.; Sivaraman, G. Turn-on fluorescence sensor for selective detection of fluoride ion and its molecular logic gates behavior. J. Mol. Liq. 2020, 317, 113913. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Dey, N. Anion binding studies with anthraimidazoledione-based positional isomers: A comprehensive analysis of different strategies for improved selectivity. Talanta 2022, 250, 123703. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Khizar, M.; Islam, M.; Hameed, A.; Moin, S.T.; Yaqub, M.; Rauf, W.; Naseer, M.M.; Ahsan, M.T.; Shafiq, Z.; et al. Synthesis of sensitive novel dual Signaling pyridopyrimidine-based fluorescent “turn off” chemosensors for anions determination. Measurement 2020, 151, 107267. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Sangtarash, S.; Sadeghi, H.; Decurtins, S.; Häner, R.; Hong, W.; Lambert, C.J.; Liu, S.-X. Probing Lewis acid–base interactions in single-molecule junctions. Nanoscale 2018, 10, 18131–18134. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, L.; Li, S.; Dong, L.; Tan, L. Anion-π Interaction in a Diketopyrrolopyrrole Derivative. Org. Lett. 2023, 25, 5774–5778. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Zhang, X.; Zuo, Y.; Tian, M.; Dong, B.; Tang, Y.; Lin, W. Triphenylamine-based silsesquioxane derivatives for multiple anion recognition via anion effect and solvent effect. Sens. Actuators B Chem. 2021, 338, 129837. [Google Scholar] [CrossRef]
- Gupta, A.S.; Paul, K.; Luxami, V. A fluorescent probe with “AIE + ESIPT” characteristics for Cu2+ and F− ions estimation. Sens. Actuators B Chem. 2017, 246, 653–661. [Google Scholar] [CrossRef]
- Fu, J.; Li, B.; Mei, H.; Chang, Y.; Xu, K. Fluorescent schiff base probes for sequential detection of Al3+ and F− and cell imaging applications. Spectrochim. Acta Part A 2020, 227, 117678. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.; Jia, Y.; Huang, L.; Wang, L.; Zhou, G.; Wang, S.; Liu, P. 1,6-Elimination reaction induced detection of fluoride ions in vitro and in vivo based on a NIR fluorescent probe. Spectrochim. Acta Part A 2019, 220, 117108. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zheng, T.; Xu, Z.; Song, Y.; Li, H.; Xia, S.; Shen, L. A novel bifunctional fluorescent and colorimetric probe for detection of mercury and fluoride ions. Spectrochim. Acta Part A 2019, 207, 88–95. [Google Scholar] [CrossRef]
- Chen, S.; Yu, H.; Zhao, C.; Hu, R.; Zhu, J.; Li, L. Indolo[3,2-b]carbazole derivative as a fluorescent probe for fluoride ion and carbon dioxide detections. Sens. Actuators B Chem. 2017, 250, 591–600. [Google Scholar] [CrossRef]
- Yang, G.; Wang, G.; Chen, K.; Yang, D. Sensing of fluoride anion based on desilylation and intramolecular charge transfer of 2-[2-(tert-butyl-diphenyl-silanyloxy)-phenyl]-4,5-diphenyl-1H-imidazole. J. Phys. Org. Chem. 2020, 34, e4162. [Google Scholar] [CrossRef]
- Kediya, S.; Manhas, A.; Jha, P.C. Benzothiazole-based chemosensor: A quick dip into its anion sensing mechanism. J. Phys. Org. Chem. 2021, 35, e4283. [Google Scholar] [CrossRef]
- Zhou, P.; Hoffmann, M.R.; Han, K.; He, G. New Insights into the Dual Fluorescence of Methyl Salicylate: Effects of Intermolecular Hydrogen Bonding and Solvation. J. Phys. Chem. B 2015, 119, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhao, L. Accurate description of excited state intramolecular proton transfer that involves zwitterionic state using optimally tuned range-separated time-dependent density functional theory. Int. J. Quantum Chem. 2018, 118, e25618. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Q.; Liu, S.; Zhao, J.; Zheng, D. Unraveling excited state dynamics and photophysical properties for a series of phenol-quinoline derivatives by controlling hydrogen bond geometry. J. Photochem. Photobiol. A 2022, 427, 113799. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, L.; Li, L.; Jin, B.; Tang, Z. Insights into Hydrogen Bonding Effect as Well as Excited State Intramolecular Proton Transfer Associated with Solvent Polarity and Atomic Electronegativity for 2-Phenyl-3-Hydroxybenzo[g]quinolone. ChemistrySelect 2023, 8, e202300933. [Google Scholar] [CrossRef]
- Zhao, J.; Song, P.; Feng, L.; Wang, X.; Tang, Z. Theoretical insights into atomic-electronegativity-regulated ESIPT behavior for B-bph-fla-OH fluorophore. J. Mol. Liq. 2023, 380, 121763. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Li, L.; Zhang, H. Theoretical Insights into Excited-State Stepwise Double Proton Transfer Associated with Solvent Polarity for 2-bis(benzothia-zolyl)naphthalene-Diol Compound. ChemistrySelect 2023, 8, e202301202. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Fan, L.; Li, F.; Song, P. Unveiling and regulating the solvent-polarity-associated excited state intramolecular double proton transfer behavior for 1-bis(benzothiazolyl)naphthalene-diol fluorophore. Spectrochim. Acta Part A 2023, 299, 122831. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Han, H.; Ding, J.; Zhou, P. Dual fluorescence of 2-(2′-hydroxyphenyl) benzoxazole derivatives via the branched decays from the upper excited-state. Phys. Chem. Chem. Phys. 2021, 23, 27304–27311. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, A.; Hao, W.; Xia, Z.; Wu, D.; Xie, P.; Lan, Y.; He, H. Unraveling the dual sensing mechanism of hydrogen peroxide probe: Photoinduced electron transfer and unusual local excited state intramolecular proton transfer. J. Lumin. 2024, 267, 120387. [Google Scholar] [CrossRef]
- Zhou, P.; Ning, C.; Alsaedi, A.; Han, K. The Effects of Heteroatoms Si and S on Tuning the Optical Properties of Rhodamine- and Fluorescein-Based Fluorescence Probes: A Theoretical Analysis. ChemPhysChem 2016, 17, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, X.; Song, L.; Zhao, J.; Tang, Z. Theoretical study on ESIPT mechanism and negative solvatochromism effect of 3-(4,5-Diphenyl-1H-imidazol-2-yl)-9-phenyl-9H-carbazol-4-ol compound in different solvents. J. Mol. Liq. 2023, 382, 122000. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, H.; Zhao, G.; Guo, Q.; Shi, W. Reversible ratiometric fluorescence probe for the detection of HClO/H2S based on excited state intramolecular proton transfer mechanism. Mol. Phys. 2023.
- Tang, Z.; Bai, T.; Zhou, P. Sensing Mechanism of a Fluorescent Probe for Cysteine: Photoinduced Electron Transfer and Invalidity of Excited-State Intramolecular Proton Transfer. J. Phys. Chem. A 2020, 124, 6920–6927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Fu, C.; Zhang, Q.; Li, S.; Ding, C. Ratiometric Fluorescent Strategy for Localizing Alkaline Phosphatase Activity in Mitochondria Based on the ESIPT Process. Anal. Chem. 2019, 91, 12377–12383. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Ding, Z.; Zhao, J.; Zheng, D. Substituent control of dynamical process for excited state intramolecular proton transfer of benzothiazole derivatives. Chem. Phys. 2022, 560, 111568. [Google Scholar] [CrossRef]
- Zhou, P.; Han, K. Unraveling the Detailed Mechanism of Excited-State Proton Transfer. Acc. Chem. Res. 2018, 51, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Huo, B.; Li, M.; Shen, A.; Bai, X.; Lai, Y.; Liu, J.; Yang, Y. A “Turn-On” fluorescent probe for sensitive and selective detection of fluoride ions based on aggregation-induced emission. RSC Adv. 2018, 8, 32497–32505. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Huang, H.; Zuo, Z.; Peng, Y. A Single Organic Fluorescent Probe for the Discrimination of Dual Spontaneous ROS in Living Organisms: Theoretical Approach. Molecules 2023, 28, 6983. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, L.; Zhou, P.; Yang, Y.; Wu, W. Distinctive fluoride fluorescent probes with ratiometric characteristics combinate desilylation, hydrogen bond and ESIPT process: Spectral and mechanistic studies. Sens. Actuators B Chem. 2018, 255, 401–407. [Google Scholar] [CrossRef]
- Karas, L.J.; Batista, P.R.; Viesser, R.V.; Tormena, C.F.; Rittner, R.; de Oliveira, P.R. Trends of intramolecular hydrogen bonding in substituted alcohols: A deeper investigation. Phys. Chem. Chem. Phys. 2017, 19, 16904–16913. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Halls, M.D.; Friesner, R.A. Highly efficient implementation of the analytical gradients of pseudospectral time-dependent density functional theory. J. Chem. Phys. 2021, 155, 024115. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Maringolo, M.P.; Tello, A.C.M.; Guimarães, A.R.; Alves, J.M.A.; das Chagas Alves Lima, F.; Longo, E.; da Silva, A.B.F. On polarization functions for Gaussian basis sets. J. Mol. Model. 2020, 26, 293. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-T.; Hsieh, C.-M. Efficient and accurate solvation energy calculation from polarizable continuum models. J. Chem. Phys. 2006, 125, 124103. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]







| HBT-Enol1 | HBT-Keto | |||
|---|---|---|---|---|
| S0 | S1 | S0 | S1 | |
| O-H1 | 0.994 | 1.017 | 1.633 | 1.871 |
| H1⋯N | 1.718 | 1.638 | 1.053 | 1.024 |
| δ(O-H1⋯N) | 147.2 | 150.2 | 139.4 | 130.9 |
| Electronic Transition | Wave Length (nm) | Energy (eV) | f a | Contrib b | CI c | Exp d (nm) | |
|---|---|---|---|---|---|---|---|
| PBT | |||||||
| Absorption | S0→S2 | 299.79 | 4.1357 | 0.0845 | H-1→L | 0.63 | ~303 |
| Emission | S0→S1 | 637.18 | 1.9458 | 0.0203 | L→H | 0.99 | |
| HBT-Enol1 | |||||||
| Absorption | S0→S1 | 332.97 | 3.7236 | 0.5117 | H→L | 0.96 | ~335 |
| HBT-Keto | |||||||
| Emission | S1→S0 | 495.85 | 2.5004 | 0.4520 | L→H | 0.99 | ~470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Lv, M.; Zhang, Y.; Gao, Y.; Cai, Z.; Zhang, Y.; Song, J.; Liu, J.; Yin, H.; Shang, F. TDDFT Study on the ESIPT Properties of 2-(2′-Hydroxyphenyl)-Benzothiazole and Sensing Mechanism of a Derived Fluorescent Probe for Fluoride Ion. Molecules 2024, 29, 1541. https://doi.org/10.3390/molecules29071541
Wang T, Lv M, Zhang Y, Gao Y, Cai Z, Zhang Y, Song J, Liu J, Yin H, Shang F. TDDFT Study on the ESIPT Properties of 2-(2′-Hydroxyphenyl)-Benzothiazole and Sensing Mechanism of a Derived Fluorescent Probe for Fluoride Ion. Molecules. 2024; 29(7):1541. https://doi.org/10.3390/molecules29071541
Chicago/Turabian StyleWang, Tingting, Meiheng Lv, Yuhang Zhang, Yue Gao, Zexu Cai, Yifan Zhang, Jiaqi Song, Jianyong Liu, Hang Yin, and Fangjian Shang. 2024. "TDDFT Study on the ESIPT Properties of 2-(2′-Hydroxyphenyl)-Benzothiazole and Sensing Mechanism of a Derived Fluorescent Probe for Fluoride Ion" Molecules 29, no. 7: 1541. https://doi.org/10.3390/molecules29071541
APA StyleWang, T., Lv, M., Zhang, Y., Gao, Y., Cai, Z., Zhang, Y., Song, J., Liu, J., Yin, H., & Shang, F. (2024). TDDFT Study on the ESIPT Properties of 2-(2′-Hydroxyphenyl)-Benzothiazole and Sensing Mechanism of a Derived Fluorescent Probe for Fluoride Ion. Molecules, 29(7), 1541. https://doi.org/10.3390/molecules29071541