Phytochemical Profile and Bioactivity of Bound Polyphenols Released from Rosa roxburghii Fruit Pomace Dietary Fiber by Solid-State Fermentation with Aspergillus niger
Abstract
:1. Introduction
2. Results and Discussion
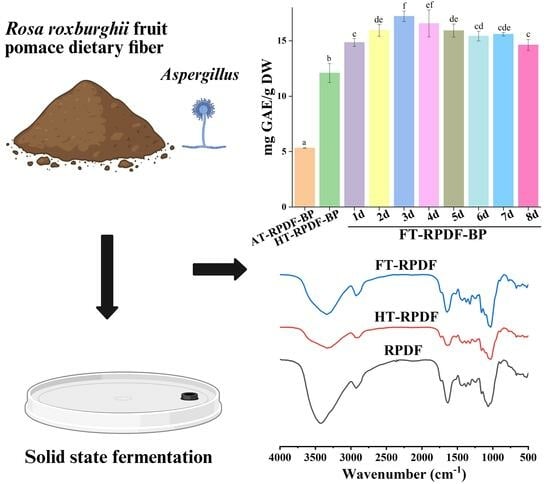
2.1. Total Phenolic Content (TPC) of AT-RPDF-BP, HT-RPDF-BP, and FT-RPDF-BP
2.2. Identification and Quantification of Bound Polyphenols
2.3. Antioxidant Activity
2.4. Inhibitory Activities of α-Glucosidase
2.5. Microstructural Comparison of RPDF, HT-RPDF, and FT-RPDF
2.6. FT-IR Changes in RPDF, HT-RPDF, and FT-RPDF
2.7. Thermal Properties of RPDF, HT-RPDF, and FT-RPDF
3. Materials and Methods
3.1. Experimental Materials
3.2. Sterilization Pretreatment of RPDF
3.3. Solid-State Fermentation of RPDF
3.4. Extraction of Bound Polyphenols
3.5. Determination of Total Phenolic Content
3.6. Quantification of Individual Phenolic Compound Contents
3.7. Determination of Antioxidant Activity
3.7.1. DPPH Radical Scavenging Activity
3.7.2. ABTS Radical Scavenging Activity
3.7.3. Oxygen Radical Absorbance Capacity (ORAC)
3.8. Determination of α-Glucosidase Inhibitory Activity
3.9. Structural Analysis
3.9.1. Surface Morphology
3.9.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.9.3. Thermos Gravimetric (TG)
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, J.; Zhang, B.; Fu, X.; Huang, Q.; Li, C.; Liu, G.; Liu, R.H. Recent advances in polysaccharides from Rose roxburghii Tratt fruits: Isolation, structural characterization, and bioactivities. Food Funct. 2022, 13, 12561–12571. [Google Scholar] [CrossRef]
- Huang, D.; Li, C.; Chen, Q.; Xie, X.; Fu, X.; Chen, C.; Huang, Q.; Huang, Z.; Dong, H. Identification of polyphenols from Rosa roxburghii Tratt pomace and evaluation of in vitro and in vivo antioxidant activity. Food Chem. 2022, 377, 131922. [Google Scholar] [CrossRef]
- Su, J.; Fu, X.; Huang, Q.; Liu, G.; Li, C. Phytochemical profile, bioactivity and prebiotic potential of bound polyphenols released from Rosa roxburghii fruit pomace dietary fiber during in vitro digestion and fermentation. Food Funct. 2022, 13, 8880–8891. [Google Scholar] [CrossRef]
- Liu, S.; Jia, M.; Chen, J.; Wan, H.; Dong, R.; Nie, S.; Xie, M.; Yu, Q. Removal of bound polyphenols and its effect on antioxidant and prebiotics properties of carrot dietary fiber. Food Hydrocoll. 2019, 93, 284–292. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, L.; Jia, X.; Liu, L.; Chi, J.; Huang, F.; Ma, Q.; Zhang, M.; Zhang, R. Bound phenolics ensure the antihyperglycemic effect of rice bran dietary fiber in db/db mice via activating the insulin signaling pathway in skeletal muscle and altering gut microbiota. J. Agric. Food Chem. 2020, 68, 4387–4398. [Google Scholar] [CrossRef]
- Khosravi, A.; Razavi, S.H.; Fadda, A.M. Advanced assessments on innovative methods to improve the bioaccessibility of polyphenols in wheat. Process Biochem. 2020, 88, 1–14. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Serwah Boateng, N.A.; Ma, H. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends Food Sci. Technol. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Li, X.; Chen, P.X.; Zhang, H.; Liu, R.; Tsao, R. Bound phenolics of quinoa seeds released by acid, alkaline, and enzymatic treatments and their antioxidant and α-glucosidase and pancreatic lipase inhibitory effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef]
- Ghosh, J.S. Solid state fermentation and food processing: A short review. Nutr. Food Sci. 2015, 6, 453. [Google Scholar] [CrossRef]
- Xie, J.; Liu, S.; Dong, R.; Xie, J.; Chen, Y.; Peng, G.; Liao, W.; Xue, P.; Feng, L.; Yu, Q. Bound polyphenols from insoluble dietary fiber of defatted rice bran by solid-state fermentation with Trichoderma viride: Profile, activity, and release mechanism. J. Agric. Food Chem. 2021, 69, 5026–5039. [Google Scholar] [CrossRef]
- Postigo, L.; Jacobo-Velázquez, D.A.; Guajardo-Flores, D.; Amezquita, L.E.G.; García-Cayuela, T. Solid-state fermentation for enhancing the nutraceutical content of agrifood by-products: Recent advances and its industrial feasibility. Food Biosci. 2021, 41, 100926. [Google Scholar]
- Song, T.; Zhang, Z.; Jin, Q.; Feng, W.; Shen, Y.; Fan, L.; Cai, W. Nutrient profiles, functional compositions, and antioxidant activities of seven types of grain fermented with Sanghuangporus sanghuang fungus. J. Food Sci. Technol. 2021, 58, 4091–4101. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, X.J.; Sun, M.B.; Li, D.; Hua, M.; Miao, X.Y.; Su, Y.; Chi, Y.P.; Wang, J.H.; Niu, H.H. Optimization of mixed fermentation conditions of dietary fiber from soybean residue and the effect on structure, properties and potential biological activity of dietary fiber from soybean residue. Molecules 2023, 28, 1322. [Google Scholar] [CrossRef]
- Liao, W.; Liu, S.; Dong, R.; Xie, J.; Chen, Y.; Hu, X.; Xie, J.; Xue, P.; Feng, L.; Yu, Q. Mixed solid-state fermentation for releasing bound polyphenols from insoluble dietary fiber in carrots via Trichoderma viride and Aspergillus niger. Food Funct. 2022, 13, 2044–2056. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Liu, R. Processed sweet corn has higher antioxidant activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef]
- Cheng, Z.; Su, L.; Moore, J.; Zhou, K.; Luther, M.; Yin, J.-J.; Lucy, Y.L. Effects of postharvest treatment and heat stress on availability of wheat antioxidants. J. Agric. Food Chem. 2006, 54, 5623–5629. [Google Scholar] [CrossRef]
- Cai, S.; Wang, O.; Wu, W.; Zhu, S.; Zhou, F.; Ji, B.; Gao, F.; Zhang, D.; Liu, J.; Cheng, Q. Comparative study of the effects of solid-state fermentation with three filamentous fungi on the total phenolics content (TPC), flavonoids, and antioxidant activities of subfractions from oats (Avena sativa L.). J. Agric. Food Chem. 2012, 60, 507–513. [Google Scholar] [CrossRef]
- Bucic-Kojic, A.; Fernandes, F.; Silva, T.; Planinic, M.; Tisma, M.; Selo, G.; Sibalic, D.; Pereira, D.M.; Andrade, P.B. Enhancement of the anti-inflammatory properties of grape pomace treated by Trametes versicolor. Food Funct. 2020, 11, 680–688. [Google Scholar] [CrossRef]
- Dhull, S.B.; Punia, S.; Kumar, R.; Kumar, M.; Nain, K.B.; Jangra, K.; Chudamani, C. Solid state fermentation of fenugreek (Trigonella foenumgraecum): Implications on bioactive compounds, mineral content and in vitro bioavailability. J. Food Sci. Technol. 2021, 58, 1927–1936. [Google Scholar] [CrossRef]
- Jose Carlos, L.M.; Leonardo, S.; Jesus, M.C.; Paola, M.R.; Alejandro, Z.C.; Juan, A.V.; Cristobal Noe, A. Solid-state fermentation with Aspergillus niger GH1 to enhance polyphenolic content and antioxidative activity of Castilla Rose (Purshia plicata). Plants 2020, 9, 1518. [Google Scholar] [CrossRef]
- Marin, L.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed]
- Saharan, P.; Sadh, P.K.; Singh Duhan, J. Comparative assessment of effect of fermentation on phenolics, flavanoids and free radical scavenging activity of commonly used cereals. Biocatal. Agric. Biotechnol. 2017, 12, 236–240. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human study and clinical trial. Crit. Rev. Food Sci. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Wang, L.; Wei, W.; Tian, X.; Shi, K.; Wu, Z. Improving bioactivities of polyphenol extracts from Psidium guajava L. leaves through co-fermentation of Monascus anka GIM 3.592 and Saccharomyces cerevisiae GIM 2.139. Ind. Crops Prod. 2016, 94, 206–215. [Google Scholar] [CrossRef]
- Alba, K.; MacNaughtan, W.; Laws, A.P.; Foster, T.J.; Campbell, G.M.; Kontogiorgos, V. Fractionation and characterisation of dietary fibre from blackcurrant pomace. Food Hydrocoll. 2018, 81, 398–408. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, Z.; Shi, X.; Zhu, Y.; Ali, F.; Dong, Y.; Zhang, F.; Zhang, B. Structural elucidation and anti-psoriasis activity of a novel polysaccharide from Saussurea Costus. Carbohyd. Polym. 2024, 333, 121963. [Google Scholar] [CrossRef]
- Gieroba, B.; Kalisz, G.; Krysa, M.; Khalavka, M.; Przekora, A. Application of vibrational spectroscopic techniques in the study of the natural polysaccharides and their cross-linking process. Int. J. Mol. Sci. 2023, 24, 2630. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, S.; Zhu, P.; Nie, C.; Ma, S.; Wang, N.; Du, X.; Zhou, Y. Purification, characterization and immunomodulatory activity of water extractable polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2017, 107, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J. Agric. Food Chem. 2012, 60, 11195–11200. [Google Scholar] [CrossRef]
- Zhai, X.; Ao, H.; Liu, W.; Zheng, J.; Li, X.; Ren, D. Physicochemical and structural properties of dietary fiber from Rosa roxburghii pomace by steam explosion. J. Food Sci. Technol. 2021, 59, 2381–2391. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wang, Y.; Liu, Z.; Ni, Y. Effects of extraction methods on the structural characteristics and functional properties of dietary fiber extracted from kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 110, 106162. [Google Scholar] [CrossRef]
- Yang, X.; Dai, J.; Zhong, Y.; Wei, X.; Wu, M.; Zhang, Y.; Huang, A.; Wang, L.; Huang, Y.; Zhang, C.; et al. Characterization of insoluble dietary fiber from three food sources and their potential hypoglycemic and hypolipidemic effects. Food Funct. 2021, 12, 6576–6587. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, F.; Xiao, Y.; Cao, J.; Wang, M.; Duan, X. Alterations in physicochemical and functional properties of buckwheat straw insoluble dietary fiber by alkaline hydrogen peroxide treatment. Food Chem. 2019, 3, 100029. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, S.; Xie, J.; Chen, Y.; Dong, R.; Zhang, X.; Liu, S.; Xie, J.; Hu, X.; Yu, Q. Antioxidant, α-amylase and α-glucosidase inhibitory activities of bound polyphenols extracted from mung bean skin dietary fiber. LWT 2020, 132, 109943. [Google Scholar] [CrossRef]





| Composition | AT-RPDF-BP | HT-RPDF-BP | FT-RPDF-BP | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1d | 2d | 3d | 4d | 5d | 6d | 7d | 8d | |||
| Gallic acid | 1.19 | 0.36 ± 0.02 | ND | ND | ND | ND | ND | ND | ND | ND |
| Gallocatechin | 0.88 ± 0.01 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Epigallcatechin | 0.24 ± 0.02 a | 0.40 ± 0.01 a | 1.06 ± 0.08 b | 1.92 ± 0.14 e | 1.56 ± 0.18 c | 1.62 ± 0.05 cd | 2.04 ± 0.05 e | 1.81 ± 0.13 de | ND | ND |
| Catechin | 0.18 ± 0.02 | 0.23 | ND | ND | ND | ND | ND | ND | ND | ND |
| Hydroxybenzoic acid | 1.18 ± 0.02 a | ND | ND | ND | 4.10 ± 0.09 b | 5.53 ± 0.03 c | 6.95 ± 0.02 d | 8.05 ± 0.01 e | 9.97 ± 0.17 g | 8.58 ± 0.04 f |
| Epicatechin | 1.36 ± 0.07 b | 0.38 ± 0.01 a | 0.37 ± 0.01 a | ND | ND | ND | ND | ND | ND | ND |
| Ellagic acid | 2.84 ± 0.08 a | 26.37 ± 0.02 h | 14.98 ± 0.02 g | 7.87 ± 0.32 f | 6.63 ± 0.22 e | 6.42 ± 0.10 e | 5.60 ± 0.02 d | 4.27 ± 0.04 b | 4.72 ± 0.01 c | 4.23 ± 0.04 b |
| Ferulic acid | 0.07 ± 0.01 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Quercetin | 0.07 a | ND | ND | ND | 0.23 ± 0.01 b | 2.29 ± 0.02 c | 3.45 ± 0.03 d | 4.09 ± 0.02 e | 5.09 ± 0.03 g | 4.59 ± 0.01 f |
| 3,4-Dihydroxy phenylpropionic acid | ND | ND | 1.41 ± 0.04 c | 1.94 ± 0.14 d | 1.33 ± 0.07 bc | 1.27 ± 0.04 b | 1.36 ± 0.04 bc | 0.91 ± 0.04 a | 0.89 ± 0.04 a | 0.80 ± 0.02 a |
| Sample | IC50 (μg/mL) |
|---|---|
| AT-RPDF-BP | 6.03 ± 0.04 d |
| HT-RPDF-BP | 4.16 ± 0.09 c |
| FT-RPDF-BP | 0.29 ± 0.01 b |
| Acarbose | 19.04 ± 0.97 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Su, J.; Zhang, Y.; Li, C.; Zhu, S. Phytochemical Profile and Bioactivity of Bound Polyphenols Released from Rosa roxburghii Fruit Pomace Dietary Fiber by Solid-State Fermentation with Aspergillus niger. Molecules 2024, 29, 1689. https://doi.org/10.3390/molecules29081689
Chen Q, Su J, Zhang Y, Li C, Zhu S. Phytochemical Profile and Bioactivity of Bound Polyphenols Released from Rosa roxburghii Fruit Pomace Dietary Fiber by Solid-State Fermentation with Aspergillus niger. Molecules. 2024; 29(8):1689. https://doi.org/10.3390/molecules29081689
Chicago/Turabian StyleChen, Qing, Juan Su, Yue Zhang, Chao Li, and Siming Zhu. 2024. "Phytochemical Profile and Bioactivity of Bound Polyphenols Released from Rosa roxburghii Fruit Pomace Dietary Fiber by Solid-State Fermentation with Aspergillus niger" Molecules 29, no. 8: 1689. https://doi.org/10.3390/molecules29081689
APA StyleChen, Q., Su, J., Zhang, Y., Li, C., & Zhu, S. (2024). Phytochemical Profile and Bioactivity of Bound Polyphenols Released from Rosa roxburghii Fruit Pomace Dietary Fiber by Solid-State Fermentation with Aspergillus niger. Molecules, 29(8), 1689. https://doi.org/10.3390/molecules29081689