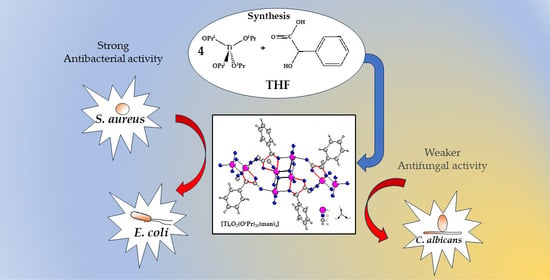
Structural Characterization and Bioactivity of a Titanium(IV)-Oxo Complex Stabilized by Mandelate Ligands
Abstract
:1. Introduction
2. Results
2.1. Structure of (1) Oxo Complex
2.2. Spectral Cheracterization of (1) and Its Composite with Poly(methyl methacrylate)
2.3. Antimicrobial Activity of (1) and Its Composites
2.4. Cytotoxicity of PMMA + (1) Composites
3. Discussion
3.1. Structure and Physicochemical Properties of (1) and Its Composite PMMA + (1)
3.2. Antimicrobial Activity of (1) and Its Composite PMMA + (1)
3.3. Cytotixity of PMMA + (1) Composites
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Ti(IV) Oxo-Complex (α-TOCs) and Preparation of PMMA/TOC Composites
4.2.1. The Synthesis of [Ti8O2(OiPr)20(man)4] (1)
4.2.2. PMMA/TOCs Composites Preparation
4.3. Analytical Methods
4.3.1. Structural and Spectroscopic Characterization of TOCs
4.3.2. Single Crystal X-ray Diffraction Measurement
4.3.3. X-ray Diffraction of Powders
4.3.4. Characterization of PMMA + TOCs Composite Materials
4.3.5. The Electron Paramagnetic Resonance (EPR) Spectroscopy
4.4. Studies of the Biological Activity of Synthesized Materials
4.4.1. Antimicrobial Activity of PMMA + (1) Composites and Powder (1)
4.4.2. Assessment of Material Cytotoxicity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhuang, G.; Yan, J.; Wen, Y.; Zhuang, Z.; Yu, Y. Two-Dimensional Transition Metal Oxides and Chalcogenides for Advanced Photocatalysis: Progress, Challenges, and Opportunities. Sol. RRL 2021, 5, 2000403. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.-J.; Kong, F.-G. Calixarene-Protected Titanium-Oxo Clusters and Their Photocurrent Responses and Photocatalytic Performances. Inorg. Chem. 2021, 60, 5034–5041. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Liang, D.; Cai, Y.; Diao, G.; Zhou, Z. A Novel Hexanuclear Titanium(IV)-Oxo-Iminodiacetate Cluster with a Ti6O9 Core: Single-Crystal Structure and Photocatalytic Activities. Dalton Trans. 2016, 45, 7581–7588. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhu, Y.-F.; Chen, Z.-H.; Liu, F.-H.; Zhao, L.; Su, Z.-M. Synthesis, Structure, and Photocatalytic Hydrogen of Three Environmentally Friendly Titanium Oxo-Clusters. Inorg. Chem. Commun. 2014, 40, 22–25. [Google Scholar] [CrossRef]
- Kubiak, B.; Piszczek, P.; Radtke, A.; Muzioł, T.; Wrzeszcz, G.; Golińska, P. Photocatalytic and Antimicrobial Activity of Titanium(IV)-Oxo Clusters of Different Core Structure. Crystals 2023, 13, 998. [Google Scholar] [CrossRef]
- Janek, M.; Muzioł, T.M.; Piszczek, P. Trinuclear Oxo-Titanium Clusters: Synthesis, Structure, and Photocatalytic Activity. Materials 2019, 12, 3195. [Google Scholar] [CrossRef] [PubMed]
- Janek, M.; Radtke, A.; Muzioł, T.; Jerzykiewicz, M.; Piszczek, P. Tetranuclear Oxo-Titanium Clusters with Different Carboxylate Aromatic Ligands: Optical Properties, DFT Calculations, and Photoactivity. Materials 2018, 11, 1661. [Google Scholar] [CrossRef] [PubMed]
- Fenton, J.L.; Laaroussi, A.; Mobian, P.; Chaumont, C.; Khalil, G.; Huguenard, C.; Henry, M. Structural Investigation of Pyridinecarboxylato Titanium(IV) Complexes: An Uncommon Monomeric Octacoordinated Complex vs. a Hexaprismatic Architecture. Eur. J. Inorg. Chem. 2014, 2014, 357–363. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Ilina, E.; Håkansson, S.; Kessler, V.G. A New Concept for Titanium Oxo-Alkoxo-Carboxylates’ Encapsulated Biocompatible Time Temperature Food Indicators Based on Arising, Not Fading Color. J. Sol.-Gel Sci. Technol. 2010, 55, 1–8. [Google Scholar] [CrossRef]
- Benedict, J.B.; Freindorf, R.; Trzop, E.; Cogswell, J.; Coppens, P. Large Polyoxotitanate Clusters: Well-Defined Models for Pure-Phase TiO2 Structures and Surfaces. J. Am. Chem. Soc. 2010, 132, 13669–13671. [Google Scholar] [CrossRef] [PubMed]
- Sokolow, J.D.; Trzop, E.; Chen, Y.; Tang, J.; Allen, L.J.; Crabtree, R.H.; Benedict, J.B.; Coppens, P. Binding Modes of Carboxylate- and Acetylacetonate-Linked Chromophores to Homodisperse Polyoxotitanate Nanoclusters. J. Am. Chem. Soc. 2012, 134, 11695–11700. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, X.; Yi, X.; Lin, X.; Zhang, J. Coordination-Delayed-Hydrolysis Method for the Synthesis and Structural Modulation of Titanium-Oxo Clusters. Acc. Chem. Res. 2022, 55, 3150–3161. [Google Scholar] [CrossRef] [PubMed]
- Svensson, F.G.; Seisenbaeva, G.A.; Kessler, V.G. Mixed-Ligand Titanium “Oxo Clusters”: Structural Insights into the Formation and Binding of Organic Molecules and Transformation into Oxide Nanostructures on Hydrolysis and Thermolysis. Eur. J. Inorg. Chem. 2017, 2017, 4117–4122. [Google Scholar] [CrossRef]
- Wu, R.-H.; Guo, M.; Yu, M.-X.; Zhu, L.-G. Two Titanium(IV)-Oxo-Clusters: Synthesis, Structures, Characterization and Recycling Catalytic Activity in the Oxygenation of Sulfides. Dalton Trans. 2017, 46, 14348–14355. [Google Scholar] [CrossRef] [PubMed]
- Czakler, M.; Artner, C.; Schubert, U. Two New Hexanuclear Titanium Oxo Cluster Types and Their Structural Connection to Known Clusters. New J. Chem. 2018, 42, 12098–12103. [Google Scholar] [CrossRef]
- Yu, Y.-Z.; Zhang, Y.-R.; Geng, C.-H.; Sun, L.; Guo, Y.; Feng, Y.-R.; Wang, Y.-X.; Zhang, X.-M. Precise and Wide-Ranged Band-Gap Tuning of Ti6-Core-Based Titanium Oxo Clusters by the Type and Number of Chromophore Ligands. Inorg. Chem. 2019, 58, 16785–16791. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-H.; Yu, Y.-Z.; Shen, Y.-H.; Yang, L.-G.; Liu, N.-N.; Zhou, Z.-Y.; Niu, Y.-S. “Three-in-One” Structural-Building-Mode-Based Ti16-Type Titanium Oxo Cluster Entirely Protected by the Ligands Benzoate and Salicylhydroxamate. Inorg. Chem. 2022, 61, 8685–8693. [Google Scholar] [CrossRef] [PubMed]
- Schubert, U. Titanium-Oxo Clusters with Bi- and Tridentate Organic Ligands: Gradual Evolution of the Structures from Small to Big. Chem. Eur. J. 2021, 27, 11239–11256. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Piszczek, P.; Muzioł, T.; Wojtczak, A. The Structural Conversion of Multinuclear Titanium(IV) μ-Oxo-Complexes. Inorg. Chem. 2014, 53, 10803–10810. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Zheng, Z.; Chen, X.-M. A Symbol Approach for Classification of Molecule-Based Magnetic Materials Exemplified by Coordination Polymers of Metal Carboxylates. Coord. Chem. Rev. 2014, 258–259, 1–15. [Google Scholar] [CrossRef]
- Rozes, L.; Sanchez, C. Titanium Oxo-Clusters: Precursors for a Lego-like Construction of Nanostructured Hybrid Materials. Chem. Soc. Rev. 2011, 40, 1006–1030. [Google Scholar] [CrossRef] [PubMed]
- Schubert, U. Chemical Modification of Titanium Alkoxides for Sol–Gel Processing. J. Mater. Chem. 2005, 15, 3701–3715. [Google Scholar] [CrossRef]
- Wang, J.-F.; Fang, W.-H.; Li, D.-S.; Zhang, L.; Zhang, J. Cocrystal of {Ti4} and {Ti6} Clusters with Enhanced Photochemical Properties. Inorg. Chem. 2017, 56, 2367–2370. [Google Scholar] [CrossRef]
- Piszczek, P.; Kubiak, B.; Golińska, P.; Radtke, A. Oxo-Titanium(IV) Complex/Polymer Composites—Synthesis, Spectroscopic Characterization and Antimicrobial Activity Test. Int. J. Mol. Sci. 2020, 21, 9663. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, B.; Radtke, A.; Topolski, A.; Wrzeszcz, G.; Golińska, P.; Kaszkowiak, E.; Sobota, M.; Włodarczyk, J.; Stojko, M.; Piszczek, P. The Composites of PCL and Tetranuclear Titanium(IV)-Oxo Complexes as Materials Exhibiting the Photocatalytic and the Antimicrobial Activity. Int. J. Mol. Sci. 2021, 22, 7021. [Google Scholar] [CrossRef]
- Cui, Y.; Zou, G.-D.; Li, H.-M.; Huang, Y.; Fan, Y. 4-Chlorosalicylate-Stabilized Titanium-Oxo Clusters with Structures Mediated by Tetrazole and Their Photophysical Properties. Polyhedron 2019, 157, 177–182. [Google Scholar] [CrossRef]
- Luo, W.; Shu, X.-P.; Liu, P.-Y.; Yu, S.-K.; Zhu, Q.-Y.; Dai, J. Lanthanide-Titanium Oxo-Clusters, New Precursors of Multifunctional Colloids for Effective Imaging and Photodynamic Therapy. J. Mol. Liq. 2020, 317, 113946. [Google Scholar] [CrossRef]
- Li, N.; Pranantyo, D.; Kang, E.-T.; Wright, D.S.; Luo, H.-K. A Simple Drop-and-Dry Approach to Grass-Like Multifunctional Nanocoating on Flexible Cotton Fabrics Using In Situ-Generated Coating Solution Comprising Titanium-Oxo Clusters and Silver Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 12093–12100. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fang, W.; Zhang, L.; Zhang, J. Atomically Precise Multimetallic Semiconductive Nanoclusters with Optical Limiting Effects. Angew. Chem. Int. Ed. 2018, 57, 11252–11256. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Hu, B.; Zhang, H.-L.; Li, C.; Shi, Y.; Li, X.; Jin, L. Antibacterial, Photothermal and Stable Ag-Titanium-Oxo-Clusters Hydrogel Designed for Wound Healing. Mater. Des. 2023, 226, 111674. [Google Scholar] [CrossRef]
- De Pasquale, I.; Lo Porto, C.; Dell’Edera, M.; Petronella, F.; Agostiano, A.; Curri, M.L.; Comparelli, R. Photocatalytic TiO2-Based Nanostructured Materials for Microbial Inactivation. Catalysts 2020, 10, 1382. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Świderski, G.; Matejczyk, M.; Lewandowski, W. Spectroscopic Characterization and Antioxidant Properties of Mandelic Acid and Its Derivatives in a Theoretical and Experimental Approach. Materials 2022, 15, 5413. [Google Scholar] [CrossRef] [PubMed]
- Egner, P.; Pavlačková, J.; Sedlaříková, J.; Pleva, P.; Mokrejš, P.; Janalíková, M. Non-Alcohol Hand Sanitiser Gels with Mandelic Acid and Essential Oils. Int. J. Mol. Sci. 2023, 24, 3855. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-C.; Yang, J.-H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-L.; Luo, W.; Wu, Y.-Y.; Su, H.-C.; Zhang, G.-L.; Zhu, Q.-Y.; Dai, J. Two Ti13-Oxo-Clusters Showing Non-Compact Structures, Film Electrode Preparation and Photocurrent Properties. Dalton Trans. 2015, 44, 19829–19835. [Google Scholar] [CrossRef]
- Ding, Q.-R.; Liu, J.-X.; Narayanam, N.; Zhang, L.; Zhang, J. Construction of Molecular Rectangles with Titanium–Oxo Clusters and Rigid Aromatic Carboxylate Ligands. Dalton Trans. 2017, 46, 16000–16003. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zou, G.-D.; Li, H.-M.; Cui, Y.; Fan, Y. A Photoactive {Ti16} Metal–Organic Capsule: Structural, Photoelectrochemical and Photocatalytic Properties. New J. Chem. 2018, 42, 14079–14082. [Google Scholar] [CrossRef]
- Kemmitt, T.; Al-Salim, N.I.; Gainsford, G.J.; Bubendorfer, A.; Waterland, M. Unprecedented Oxo-Titanium Citrate Complex Precipitated from Aqueous Citrate Solutions, Exhibiting a Novel Bilayered Ti8O10 Structural Core. Inorg. Chem. 2004, 43, 6300–6306. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Dadzie, O.E.; Galadari, H. Chemical Peeling in Ethnic Skin: An Update. Br. J. Dermatol. 2013, 169 (Suppl. S3), 82–90. [Google Scholar] [CrossRef] [PubMed]
- Gentili, G.; Perugini, P.; Bugliaro, S.; D’Antonio, C. Efficacy and Safety of a New Peeling Formulated with a Pool of PHAs for the Treatment of All Skin Types, Even Sensitive. J. Cosmet. Dermatol. 2023, 22, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Dębowska, R.M.; Kaszuba, A.; Michalak, I.; Dzwigałowska, A.; Cieścińska, C.; Jakimiuk, E.; Zielińska, J.; Kaszuba, A. Evaluation of the Efficacy and Tolerability of Mandelic Acid-Containing Cosmetic Formulations for Acne Skin Care. Dermatol. Rev./Przegląd Dermatol. 2015, 4, 316–321. [Google Scholar] [CrossRef]
- Greive, K.; Tran, D.; Townley, J.; Barnes, T. An Antiaging Skin Care System Containing Alpha Hydroxy Acids and Vitamins Improves the Biomechanical Parameters of Facial Skin. CCID 2014, 8, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Świsłocka, R.; Świderski, G.; Nasiłowska, J.; Sokołowska, B.; Wojtczak, A.; Lewandowski, W. Research on the Electron Structure and Antimicrobial Properties of Mandelic Acid and Its Alkali Metal Salts. Int. J. Mol. Sci. 2023, 24, 3078. [Google Scholar] [CrossRef] [PubMed]
- Masoud, M.S.; Ali, A.E.; Shokry, A.A.; Kolkaila, S.A. Chelation and Molecular Structure of Mandelic Acid Complexes. J. Chem. Res. Adv. 2021, 2, 1–9. [Google Scholar]
- Youzhu, Y.; Hui, W.; Leilei, L.; Yuhua, G.; Jing, F.; Yichao, L. Crystal Structure of Bis(μ2-2-Oxido-2-Phenylacetato-κ3 O,O′:O′)-Bis(N-Oxido-Benzamide-κ2 O,O′)-Bis(Propan-2-Olato-κ1 O)Dititanium(IV), C36H38N2O12Ti2. Z. Krist. New Cryst. Struct. 2022, 237, 957–959. [Google Scholar] [CrossRef]
- Youzhu, Y.; Yuhua, G.; Yongsheng, N.; Nana, L.; Hongfei, Z. Crystal Structure of Bis(μ2-2-Oxido-2-Phenylacetate-κ3 O:O,O′)-Bis(1-Isopropoxy-2-Oxo-2-Phenylethan-1-Olato-κ2 O,O′)-Bis(Propan-2-Olato-κ1 O)Dititanium(IV), C44H52O14Ti2. Z. Krist. New Cryst. Struct. 2021, 236, 467–469. [Google Scholar] [CrossRef]
- Schetter, B.; Stosiek, C.; Ziemer, B.; Mahrwald, R. Multinuclear Enantiopure Titanium Self-Assembly Complexes—Synthesis, Characterization and Application to Organic Synthesis. Appl. Organometal. Chem. 2007, 21, 139–145. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- ISO 22196:2011 Standard; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/54431.html (accessed on 25 November 2023).
- Cicco, S.; Vona, D.; Gristina, R.; Sardella, E.; Ragni, R.; Lo Presti, M.; Farinola, G. Biosilica from Living Diatoms: Investigations on Biocompatibility of Bare and Chemically Modified Thalassiosira Weissflogii Silica Shells. Bioengineering 2016, 3, 35. [Google Scholar] [CrossRef]
- Xue, F.; Janzen, D.M.; Knecht, D.A. Contribution of Filopodia to Cell Migration: A Mechanical Link between Protrusion and Contraction. Int. J. Cell Biol. 2010, 2010, 507821. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Richert, M.; Grodzicki, A.; Głowiak, T.; Wojtczak, A. Synthesis, Crystal Structures and Spectroscopic Characterization of [Ti8O8(OOCR)16] (Where R = But, CH2But, C(CH3)2Et). Polyhedron 2005, 24, 663–670. [Google Scholar] [CrossRef]
- Frot, T.; Cochet, S.; Laurent, G.; Sassoye, C.; Popall, M.; Sanchez, C.; Rozes, L. Ti8O8(OOCR)16, a New Family of Titanium–Oxo Clusters: Potential NBUs for Reticular Chemistry. Eur. J. Inorg. Chem. 2010, 2010, 5650–5659. [Google Scholar] [CrossRef]
- Fan, X.; Yuan, F.; Li, D.; Chen, S.; Cheng, Z.; Zhang, Z.; Xiang, S.; Zang, S.; Zhang, J.; Zhang, L. Threefold Collaborative Stabilization of Ag14-Nanorods by Hydrophobic Ti16-Oxo Clusters and Alkynes: Designable Assembly and Solid-State Optical-Limiting Application. Angew. Chem. Int. Ed. 2021, 60, 12949–12954. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Reinsch, H.; Heymans, N.; Wahiduzzaman, M.; Martineau-Corcos, C.; De Weireld, G.; Maurin, G.; Serre, C. Toward a Rational Design of Titanium Metal-Organic Frameworks. Matter 2020, 2, 440–450. [Google Scholar] [CrossRef]
- Kim, B.; Keum, Y.; Chen, Y.-P.; Oh, H.S.; Lee, J.Y.; Park, J. Stimuli-Responsive Ti-Organic Gels and Aerogels Derived from Ti-Oxo Clusters: Hierarchical Porosity and Photocatalytic Activity. Inorg. Chem. 2019, 58, 15936–15941. [Google Scholar] [CrossRef] [PubMed]
- Czakler, M.; Artner, C.; Schubert, U. Influence of the Phosphonate Ligand on the Structure of Phosphonate-Substituted Titanium Oxo Clusters. Eur. J. Inorg. Chem. 2013, 2013, 5790–5796. [Google Scholar] [CrossRef]
- Fan, X.; Fu, H.; Gao, M.-Y.; Zhang, L.; Zhang, J. One-Pot and Postsynthetic Phenol-Thermal Synthesis toward Highly Stable Titanium-Oxo Clusters. Inorg. Chem. 2019, 58, 13353–13359. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Gao, M.-Y.; Fang, W.-H.; Zhang, L.; Zhang, J. Bandgap Engineering of Titanium-Oxo Clusters: Labile Surface Sites Used for Ligand Substitution and Metal Incorporation. Angew. Chem. Int. Ed. 2016, 55, 5160–5165. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Luo, W.; Wang, Y.-H.; Pu, Y.-Y.; Zhang, X.; You, L.-S.; Zhu, Q.-Y.; Dai, J. Titanium–Oxo–Clusters with Dicarboxylates: Single-Crystal Structure and Photochromic Effect. Inorg. Chem. 2012, 51, 8982–8988. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, Y.; Wamer, W.G.; Yin, J.-J. Electron Spin Resonance Spectroscopy for the Study of Nanomaterial-Mediated Generation of Reactive Oxygen Species. J. Food Drug Anal. 2014, 22, 49–63. [Google Scholar] [CrossRef]
- Xiong, L.-B.; Li, J.-L.; Yang, B.; Yu, Y. Ti3+ in the Surface of Titanium Dioxide: Generation, Properties and Photocatalytic Application. J. Nanomater. 2012, 2012, 831524. [Google Scholar] [CrossRef]
- Suriye, K.; Lobo-Lapidus, R.J.; Yeagle, G.J.; Praserthdam, P.; Britt, R.D.; Gates, B.C. Probing Defect Sites on TiO2 with [Re3(CO)12H3]: Spectroscopic Characterization of the Surface Species. Chem. Eur. J. 2008, 14, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Férey, G. A New Photoactive Crystalline Highly Porous Titanium(IV) Dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.; Murphy, D.M.; Che, M. An EPR Characterisation of Stable and Transient Reactive Oxygen Species Formed under Radiative and Non-Radiative Conditions. Res. Chem. Intermed. 2019, 45, 5763–5779. [Google Scholar] [CrossRef]
- Śmigiel, J.; Piszczek, P.; Wrzeszcz, G.; Jędrzejewski, T.; Golińska, P.; Radtke, A. The Composites of PCL and Tetranuclear Titanium(IV)–Oxo Complex with Acetylsalicylate Ligands—Assessment of Their Biocompatibility and Antimicrobial Activity with the Correlation to EPR Spectroscopy. Materials 2023, 16, 297. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, A.; Grohmann, E. Antimicrobials Functioning through ROS-Mediated Mechanisms: Current Insights. Microorganisms 2021, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P.; Skiba-Kurek, I.; Mrowiec, P.; Karczewska, E.; Drożdż, R. Synergistic ROS-Associated Antimicrobial Activity of Silver Nanoparticles and Gentamicin against Staphylococcus epidermidis. Int. J. Nanomed. 2020, 15, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.-L.; Wong, R.S.-M.; Lam, K.-H.; Hung, L.-K.; Wong, M.-M.; Yung, L.-H.; Ho, Y.-W.; Wong, W.-Y.; Hau, D.K.-P.; Gambari, R.; et al. The Role of Reactive Oxygen Species in the Biological Activity of Antimicrobial Agents: An Updated Mini Review. Chem.-Biol. Interact. 2020, 320, 109023. [Google Scholar] [CrossRef]
- Joe, A.; Park, S.-H.; Kim, D.-J.; Lee, Y.-J.; Jhee, K.-H.; Sohn, Y.; Jang, E.-S. Antimicrobial Activity of ZnO Nanoplates and Its Ag Nanocomposites: Insight into an ROS-Mediated Antibacterial Mechanism under UV Light. J. Solid. State Chem. 2018, 267, 124–133. [Google Scholar] [CrossRef]
- Beyene, B.B.; Mihirteu, A.M.; Ayana, M.T.; Yibeltal, A.W. Synthesis, Characterization and Antibacterial Activity of Metalloporphyrins: Role of Central Metal Ion. Results Chem. 2020, 2, 100073. [Google Scholar] [CrossRef]
- Chen, S.; Wu, G.; Zeng, H. Preparation of High Antimicrobial Activity Thiourea Chitosan–Ag+ Complex. Carbohydr. Polym. 2005, 60, 33–38. [Google Scholar] [CrossRef]
- Burns, J.; McCoy, C.P.; Irwin, N.J. Synergistic Activity of Weak Organic Acids against Uropathogens. J. Hosp. Infect. 2021, 111, 78–88. [Google Scholar] [CrossRef]
- Tshuva, E.Y.; Peri, D. Modern Cytotoxic Titanium(IV) Complexes; Insights on the Enigmatic Involvement of Hydrolysis. Coord. Chem. Rev. 2009, 253, 2098–2115. [Google Scholar] [CrossRef]
- Immel, T.A.; Grützke, M.; Batroff, E.; Groth, U.; Huhn, T. Cytotoxic Dinuclear Titanium-Salan Complexes: Structural and Biological Characterization. J. Inorg. Biochem. 2012, 106, 68–75. [Google Scholar] [CrossRef] [PubMed]
- CrysAlis CCD. CrysAlis Red and CrysAlis CCD; Oxford Diffraction Ltd.: Oxfordshire, UK, 2000. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K.; Berndt, M. Diamond, Release 2.1e; Crystal Impact GbR: Bonn, Germany, 2001. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]








| Composite | C | O | Al | Ti |
|---|---|---|---|---|
| PMMA | 26.10 | 72.23 | 1.67 | - |
| PMMA + (1) 2 wt.% | 28.27 | 71.00 | 0.45 | 0.27 |
| PMMA + (1) 5 wt.% | 24.75 | 66.45 | 0.59 | 8.21 |
| PMMA + (1) 10 wt.% | 19.02 | 67.24 | 0.52 | 13.22 |
| PMMA + (1) 20 wt.% | 18.77 | 58.68 | 0.48 | 22.07 |
| Sample | g-Factor | Species |
|---|---|---|
| (1) | 2.025, 2.011, 2.003 | O2− |
| 1.992 | Ti(III) | |
| PMMA | - | - |
| PMMA + (1) 5 wt.% | 2.010, 2.002 | O2− |
| 2.005, 2.000 | O− | |
| 1.992 | Ti(III) | |
| PMMA + (1) 10 wt.% | 2.025, 2.010, 2.000 | O2− |
| 2.016 | O− | |
| 1.992 | Ti(III) | |
| PMMA + (1) 20 wt.% | 2.025, 2.010, 2.002 | O2− |
| 2.016, 2.005, 2.000 | O− | |
| 1.992, 1.972 | Ti(III) |
| No. | Microorganisms | |||||
|---|---|---|---|---|---|---|
| Samples | E. coli ATCC 8739 | E. coli ATCC 25922 | S. aureus ATCC 6538 | S. aureus ATCC 25923 | C. albicans ATCC 10231 | |
| 1 | (1) 2 wt.% | 6.0 (>99.99%) | 6.0 (>99.99%) | 4.2 (>99.99%) | 5.4 (>99.99%) | 0 (0%) |
| 2 | (1) 5 wt.% | 6.0 (>99.99%) | 5.7 (>99.99%) | 5.9 (>99.99%) | 6.0 (>99.99%) | 0.3 (53.20%) |
| 3 | (1) 10 wt.% | 6.0 (>99.99%) | 6.0 (>99.99%) | 6.2 (>99.99%) | 6.0 (>99.99%) | 0.9 (87.45%) |
| 4 | (1) 20 wt.% | 6.0 (>99.99%) | 6.0 (>99.99%) | 6.5 (>99.99%) | 5.7 (>99.99%) | 6.7 (>99.99%) |
| 5 | PMMA | none (0%) | none (0%) | none (0%) | none (0%) | none (0%) |
| 6 | PMMA + (1) 2 wt.% | 2.0 (99.00%) | 3.1 (>99.90%) | 4.7 (>99.99%) | 5.1 (>99.99%) | +0.9 (+87.41%) |
| 7 | PMMA + (1) 5 wt.% | 3.1 (>99.90%) | 4.9 (>99.99%) | 4.7 (>99.99%) | 5.1 (>99.99%) | +0.82 (+84.86%) |
| 8 | PMMA + (1) 10 wt.% | 4.9 (>99.99%) | 3.9 (>99.90%) | 4.7 (>99.99%) | 5.1 (>99.99%) | +0.8 (+84.20%) |
| 9 | PMMA + (1) 20 wt.% | 4.9 (>99.99%) | 4.7 (>99.99%) | 4.7 (>99.99%) | 5.1 (>99.99%) | +0.7 (+80.05%) |
| Empirical formula | C92H164O34Ti8 (1) |
| Formula weight | 2197.42 |
| Temperature | 100(2) K |
| Wavelength [Å] | 1.54184 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| Unit cell dimensions [Å] and [°] | a = 19.9172(6) |
| b = 12.3935(3) | |
| c = 24.4651(8) | |
| α = 90 | |
| β = 111.483(4) | |
| γ = 90 | |
| Volume [Å3] | 5619.5(3) |
| Z, calculated density [Mg/m3] | 2, 1.299 |
| Absorption coefficient [mm−1] | 5.193 |
| F(000) | 2328 |
| Crystalsize [mm3] | 0.220 × 0.180 × 0.080 |
| Theta range for data collection [°] | 2.384 to 74.492 |
| Index ranges | −24 ≤ h ≤ 24 |
| −15 ≤ k ≤ 14 | |
| −30 ≤ l ≤ 21 | |
| Reflections collected/unique | 44,138/11,218 [R(int) = 0.0943 |
| Completeness to theta | 67.684° 99.9% |
| Absorption correction | Gaussian |
| Max. and min. transmission | 1.000 and 0.414 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 11,218/35/654 |
| Goodness-of-fit on F2 | 1.054 |
| Final R indices [I > 2sigma(I)] | R1 a = 0.0865, wR2 b = 0.2451 |
| R indices (all data) | R1 a = 0.1092, wR2 b = 0.2674 |
| Largest diff. peak and hole | 0.766 and −0.915 e·Å−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, B.; Muzioł, T.; Wrzeszcz, G.; Radtke, A.; Golińska, P.; Jędrzejewski, T.; Wrotek, S.; Piszczek, P. Structural Characterization and Bioactivity of a Titanium(IV)-Oxo Complex Stabilized by Mandelate Ligands. Molecules 2024, 29, 1736. https://doi.org/10.3390/molecules29081736
Kubiak B, Muzioł T, Wrzeszcz G, Radtke A, Golińska P, Jędrzejewski T, Wrotek S, Piszczek P. Structural Characterization and Bioactivity of a Titanium(IV)-Oxo Complex Stabilized by Mandelate Ligands. Molecules. 2024; 29(8):1736. https://doi.org/10.3390/molecules29081736
Chicago/Turabian StyleKubiak, Barbara, Tadeusz Muzioł, Grzegorz Wrzeszcz, Aleksandra Radtke, Patrycja Golińska, Tomasz Jędrzejewski, Sylwia Wrotek, and Piotr Piszczek. 2024. "Structural Characterization and Bioactivity of a Titanium(IV)-Oxo Complex Stabilized by Mandelate Ligands" Molecules 29, no. 8: 1736. https://doi.org/10.3390/molecules29081736
APA StyleKubiak, B., Muzioł, T., Wrzeszcz, G., Radtke, A., Golińska, P., Jędrzejewski, T., Wrotek, S., & Piszczek, P. (2024). Structural Characterization and Bioactivity of a Titanium(IV)-Oxo Complex Stabilized by Mandelate Ligands. Molecules, 29(8), 1736. https://doi.org/10.3390/molecules29081736