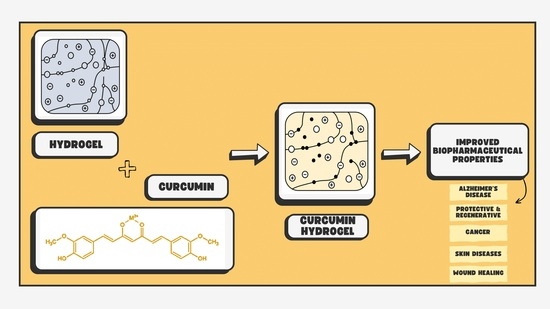
Wondrous Yellow Molecule: Are Hydrogels a Successful Strategy to Overcome the Limitations of Curcumin?
Abstract
:1. Introduction
1.1. Curcumin
1.2. Hydrogels
2. Use of Hydrogels for Curcumin Delivery
2.1. Hydrogels’ Releasing Modes for Curcumin Delivery
2.2. Wound Healing
2.3. Skin Diseases
2.4. Cancer
2.5. Alzheimer’s Disease
2.6. Protective and Regenerative Properties
3. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yun, D.G.; Lee, D.G. Antibacterial Activity of Curcumin via Apoptosis-like Response in Escherichia Coli. Appl. Microbiol. Biotechnol. 2016, 100, 5505–5514. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, T.; Farooqui, A.A. Curcumin: Historical Background, Chemistry, Pharmacological Action, and Potential Therapeutic Value. In Curcumin for Neurological and Psychiatric Disorders; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–44. [Google Scholar] [CrossRef]
- Bhatia, M.; Bhalerao, M.; Cruz-Martins, N.; Kumar, D. Curcumin and Cancer Biology: Focusing Regulatory Effects in Different Signalling Pathways. Phytother. Res. 2021, 35, 4913–4929. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an Active Component of Turmeric (Curcuma Longa ), and Its Effects on Health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Marton, L.T.; Barbalho, S.M.; Sloan, K.P.; Sloan, L.A.; Goulart, R.d.A.; Araújo, A.C.; Bechara, M.D. Curcumin, Autoimmune and Inflammatory Diseases: Going beyond Conventional Therapy—A Systematic Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2140–2157. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Mukai, S.; Yamada, S.; Murata, S.; Yabumoto, H.; Maeda, T.; Akamatsu, S. The Efficacy and Safety of Highly-Bioavailable Curcumin for Treating Knee Osteoarthritis: A 6-Month Open-Labeled Prospective Study. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2020, 13, 117954412094847. [Google Scholar] [CrossRef]
- Alves, C.; Ribeiro, A.; Pinto, E.; Santos, J.; Soares, G. Exploring Z-Tyr-Phe-OH-Based Hydrogels Loaded with Curcumin for the Development of Dressings for Wound Healing. J. Drug Deliv. Sci. Technol. 2022, 73, 103484. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a Photosensitizer: From Molecular Structure to Recent Advances in Antimicrobial Photodynamic Therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological Activities of Curcuminoids, Other Biomolecules from Turmeric and Their Derivatives—A Review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Jyotirmayee, B.; Mahalik, G. A Review on Selected Pharmacological Activities of Curcuma Longa L. Int. J. Food Prop. 2022, 25, 1377–1398. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin-From Molecule to Biological Function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Iglesias, O.; Naidoo, V.; Carrera, I.; Corzo, L.; Cacabelos, R. Natural Bioactive Products as Epigenetic Modulators for Treating Neurodegenerative Disorders. Pharmaceuticals 2023, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef] [PubMed]
- Pancholi, V.; Smina, T.P.; Kunnumakkara, A.B.; Maliakel, B.; Krishnakumar, I.M. Safety Assessment of a Highly Bioavailable Curcumin-Galactomannoside Complex (CurQfen) in Healthy Volunteers, with a Special Reference to the Recent Hepatotoxic Reports of Curcumin Supplements: A 90-Days Prospective Study. Toxicol. Rep. 2021, 8, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Redaelli, F.; Sorbona, M.; Rossi, F. Synthesis and Processing of Hydrogels for Medical Applications. In Bioresorbable Polymers for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 205–228. [Google Scholar] [CrossRef]
- Bercea, M. Bioinspired Hydrogels as Platforms for Life-Science Applications: Challenges and Opportunities. Polymers 2022, 14, 2365. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional Hydrogels With Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive Hydrogels for Bone Regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Attia, L.; Chen, L.; Doyle, P.S. Orthogonal Gelations to Synthesize Core–Shell Hydrogels Loaded with Nanoemulsion-Templated Drug Nanoparticles for Versatile Oral Drug Delivery. Adv. Healthc. Mater. 2023, 12, 2301667. [Google Scholar] [CrossRef] [PubMed]
- Völlmecke, K.; Afroz, R.; Bierbach, S.; Brenker, L.J.; Frücht, S.; Glass, A.; Giebelhaus, R.; Hoppe, A.; Kanemaru, K.; Lazarek, M.; et al. Hydrogel-Based Biosensors. Gels 2022, 8, 768. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Pedrares, N.; Fuentes-Boquete, I.; Díaz-Prado, S.; Rey-Rico, A. Hydrogel-Based Localized Nonviral Gene Delivery in Regenerative Medicine Approaches—An Overview. Pharmaceutics 2020, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Cai, J.; Cheng, H.; Wang, W. Sustained Release of Therapeutic Gene by Injectable Hydrogel for Hepatocellular Carcinoma. Int. J. Pharm. X 2023, 6, 100195. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Cai, Y.; Zhi, Z.; Guo, Q.; Mao, L.; Gao, Y.; Yuan, F.; Van Der Meeren, P. Assembly of Propylene Glycol Alginate/β-Lactoglobulin Composite Hydrogels Induced by Ethanol for Co-Delivery of Probiotics and Curcumin. Carbohydr. Polym. 2021, 254, 117446. [Google Scholar] [CrossRef] [PubMed]
- Udeni Gunathilake, T.; Ching, Y.; Chuah, C. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, M.; Sánchez-Espejo, R.; Casula, L.; Barbosa, R.D.M.; Sandri, G.; Cardia, M.C.; Lai, F.; Viseras, C. Clay-Based Hydrogels as Drug Delivery Vehicles of Curcumin Nanocrystals for Topical Application. Pharmaceutics 2022, 14, 2836. [Google Scholar] [CrossRef]
- Shahbazizadeh, S.; Naji-Tabasi, S.; Shahidi-Noghabi, M. Development of Soy Protein/Sodium Alginate Nanogel-Based Cress Seed Gum Hydrogel for Oral Delivery of Curcumin. Chem. Biol. Technol. Agric. 2022, 9, 41. [Google Scholar] [CrossRef]
- Faris, T.M.; Ahmad, A.M.; Mohammed, H.A.; Abdullah Alamoudi, J.; Alsunbul, M.; Alrashidi, A.; Abdullah, O.; Altwaijry, N.; Hassan, A.S. Preparation and Evaluation of Transdermal Hydrogel of Chitosan Coated Nanocurcumin for Enhanced Stability and Skin Permeability. Arab. J. Chem. 2023, 16, 105302. [Google Scholar] [CrossRef]
- Ning, P.; Lü, S.; Bai, X.; Wu, X.; Gao, C.; Wen, N.; Liu, M. High Encapsulation and Localized Delivery of Curcumin from an Injectable Hydrogel. Mater. Sci. Eng. C 2018, 83, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hoang Thi, T.T.; Lee, Y.; Ryu, S.B.; Sung, H.-J.; Park, K.D. Oxidized Cyclodextrin-Functionalized Injectable Gelatin Hydrogels as a New Platform for Tissue-Adhesive Hydrophobic Drug Delivery. RSC Adv. 2017, 7, 34053–34062. [Google Scholar] [CrossRef]
- Babić, M.M.; Vukomanović, M.; Stefanič, M.; Nikodinović-Runić, J.; Tomić, S.L. Controlled Curcumin Release from Hydrogel Scaffold Platform Based on 2-Hydroxyethyl Methacrylate/Gelatin/Alginate/Iron(III) Oxide. Macro Chem. Phys. 2020, 221, 2000186. [Google Scholar] [CrossRef]
- Ayar, Z.; Shafieian, M.; Mahmoodi, N.; Sabzevari, O.; Hassannejad, Z. A Rechargeable Drug Delivery System Based on pNIPAM Hydrogel for the Local Release of Curcumin. J. Appl. Polym. Sci. 2021, 138, 51167. [Google Scholar] [CrossRef]
- Caldas, B.S.; Nunes, C.S.; Panice, M.R.; Scariot, D.B.; Nakamura, C.V.; Muniz, E.C. Manufacturing Micro/Nano Chitosan/Chondroitin Sulfate Curcumin-Loaded Hydrogel in Ionic Liquid: A New Biomaterial Effective against Cancer Cells. Int. J. Biol. Macromol. 2021, 180, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhi, F.; Jia, X.; Zhang, X.; Ambardekar, R.; Meng, Z.; Paradkar, A.R.; Hu, Y.; Yang, Y. Enhanced Brain Targeting of Curcumin by Intranasal Administration of a Thermosensitive Poloxamer Hydrogel. J. Pharm. Pharmacol. 2013, 65, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-J.; Liu, X.-Y.; Tang, L.-M.-Y.; Li, P.-F.; Qiu, F.; Yang, A.-H. Anti-Depressant Effect of Curcumin-Loaded Guanidine-Chitosan Thermo-Sensitive Hydrogel by Nasal Delivery. Pharm. Dev. Technol. 2020, 25, 316–325. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.C.; De Lima, G.R.F.; Klein, R.S.; Souza, P.R.; Vilsinski, B.H.; Garcia, F.P.; Nakamura, C.V.; Martins, A.F. Thermo-and pH-Responsive Chitosan/Gellan Gum Hydrogels Incorporated with the β-Cyclodextrin/Curcumin Inclusion Complex for Efficient Curcumin Delivery. React. Funct. Polym. 2021, 165, 104955. [Google Scholar] [CrossRef]
- Ghaffari, R.; Eslahi, N.; Tamjid, E.; Simchi, A. Dual-Sensitive Hydrogel Nanoparticles Based on Conjugated Thermoresponsive Copolymers and Protein Filaments for Triggerable Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 19336–19346. [Google Scholar] [CrossRef]
- Kumar, B.A.; Nayak, R.R. Supramolecular Phenoxy-Alkyl Maleate-Based Hydrogels and Their Enzyme/pH-Responsive Curcumin Release. New J. Chem. 2019, 43, 5559–5567. [Google Scholar] [CrossRef]
- Puiggalí-Jou, A.; Cazorla, E.; Ruano, G.; Babeli, I.; Ginebra, M.-P.; García-Torres, J.; Alemán, C. Electroresponsive Alginate-Based Hydrogels for Controlled Release of Hydrophobic Drugs. ACS Biomater. Sci. Eng. 2020, 6, 6228–6240. [Google Scholar] [CrossRef] [PubMed]
- Postolović, K.; Ljujić, B.; Kovačević, M.M.; Đorđević, S.; Nikolić, S.; Živanović, S.; Stanić, Z. Optimization, Characterization, and Evaluation of Carrageenan/Alginate/Poloxamer/Curcumin Hydrogel Film as a Functional Wound Dressing Material. Mater. Today Commun. 2022, 31, 103528. [Google Scholar] [CrossRef]
- Cheng, F.; Yi, X.; Dai, J.; Fan, Z.; He, J.; Huang, Y.; Li, H. Photothermal MXene@Zn-MOF-Decorated Bacterial Cellulose-Based Hydrogel Wound Dressing for Infectious Wound Healing. Cell Rep. Phys. Sci. 2023, 4, 101619. [Google Scholar] [CrossRef]
- Gupta, A.; Keddie, D.J.; Kannappan, V.; Gibson, H.; Khalil, I.R.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. Production and Characterisation of Bacterial Cellulose Hydrogels Loaded with Curcumin Encapsulated in Cyclodextrins as Wound Dressings. Eur. Polym. J. 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Aldakheel, F.M.; Mohsen, D.; El Sayed, M.M.; Fagir, M.H.; El Dein, D.K. Employing of Curcumin–Silver Nanoparticle-Incorporated Sodium Alginate-Co-Acacia Gum Film Hydrogels for Wound Dressing. Gels 2023, 9, 780. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Lin, C.; Yuan, Z.; He, Y.; Chen, M.; Li, K.; Hu, J.; Yang, Y.; Xia, Z.; Cai, K. Near Infrared Light-Triggered on-Demand Cur Release from Gel-PDA@Cur Composite Hydrogel for Antibacterial Wound Healing. Chem. Eng. J. 2021, 403, 126182. [Google Scholar] [CrossRef]
- Le, T.T.N.; Nguyen, T.K.N.; Nguyen, V.M.; Dao, T.C.M.; Nguyen, H.B.C.; Dang, C.T.; Le, T.B.C.; Nguyen, T.K.L.; Nguyen, P.T.T.; Dang, L.H.N.; et al. Development and Characterization of a Hydrogel Containing Curcumin-Loaded Nanoemulsion for Enhanced In Vitro Antibacteria and In Vivo Wound Healing. Molecules 2023, 28, 6433. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.-G.; Lee, B.-T. Curcumin Incorporation into an Oxidized Cellulose Nanofiber-Polyvinyl Alcohol Hydrogel System Promotes Wound Healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Cardoso-Daodu, I.M.; Ilomuanya, M.O.; Azubuike, C.P. Development of Curcumin-Loaded Liposomes in Lysine–Collagen Hydrogel for Surgical Wound Healing. Beni-Suef Univ. J. Basic. Appl. Sci. 2022, 11, 100. [Google Scholar] [CrossRef]
- Kiti, K.; Suwantong, O. Bilayer Wound Dressing Based on Sodium Alginate Incorporated with Curcumin-β-Cyclodextrin Inclusion Complex/Chitosan Hydrogel. Int. J. Biol. Macromol. 2020, 164, 4113–4124. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Kumar, S.; Raut, J.; Singh, M.; Kaur, S.; Sharma, G.; Roldan, T.L.; Trehan, S.; Holloway, J.; Wahler, G.; et al. Systematic Development and Characterization of Novel, High Drug-Loaded, Photostable, Curcumin Solid Lipid Nanoparticle Hydrogel for Wound Healing. Antioxidants 2021, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Bibi, S.; Mohanta, Y.K.; Kumar Mohanta, T.; Kumar, S.; Singh, I.; Saad Khan, M.; Ranjan Rauta, P.; Alshammari, A.; Alharbi, M.; et al. In Vitro and In Silico Characterization of Curcumin-Loaded Chitosan–PVA Hydrogels: Antimicrobial and Potential Wound Healing Activity. Gels 2023, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Jiao, H.; Tian, Y.; Zhao, L.; Liao, X.; Fan, Z.; Liu, B. Facile and Large-Scale Synthesis of Curcumin/PVA Hydrogel: Effectively Kill Bacteria and Accelerate Cutaneous Wound Healing in the Rat. J. Biomater. Sci. Polym. Ed. 2018, 29, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, X.; Huang, X.; Chen, L. Bioinspired Nanovesicles Released from Injectable Hydrogels Facilitate Diabetic Wound Healing by Regulating Macrophage Polarization and Endothelial Cell Dysfunction. J. Nanobiotechnol. 2023, 21, 358. [Google Scholar] [CrossRef] [PubMed]
- Kamar, S.S.; Abdel-Kader, D.H.; Rashed, L.A. Beneficial Effect of Curcumin Nanoparticles-Hydrogel on Excisional Skin Wound Healing in Type-I Diabetic Rat: Histological and Immunohistochemical Studies. Ann. Anat.—Anat. Anz. 2019, 222, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Samanta, S.; Kundu, P.P. Curcumin Entrapped Gelatin/Ionically Modified Bacterial Cellulose Based Self-Healable Hydrogel Film: An Eco-Friendly Sustainable Synthesis Method of Wound Healing Patch. Int. J. Biol. Macromol. 2019, 122, 940–953. [Google Scholar] [CrossRef]
- Farazin, A.; Mohammadimehr, M.; Ghasemi, A.H.; Naeimi, H. Design, Preparation, and Characterization of CS/PVA/SA Hydrogels Modified with Mesoporous Ag2O/SiO2 and Curcumin Nanoparticles for Green, Biocompatible, and Antibacterial Biopolymer Film. RSC Adv. 2021, 11, 32775–32791. [Google Scholar] [CrossRef]
- Deka, C.; Deka, D.; Bora, M.M.; Jha, D.K.; Kakati, D.K. Investigation of pH-Sensitive Swelling and Curcumin Release Behavior of Chitglc Hydrogel. J. Polym. Environ. 2018, 26, 4034–4045. [Google Scholar] [CrossRef]
- Pham, L.; Dang, L.H.; Truong, M.D.; Nguyen, T.H.; Le, L.; Le, V.T.; Nam, N.D.; Bach, L.G.; Nguyen, V.T.; Tran, N.Q. A Dual Synergistic of Curcumin and Gelatin on Thermal-Responsive Hydrogel Based on Chitosan-P123 in Wound Healing Application. Biomed. Pharmacother. 2019, 117, 109183. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.H.; Huynh, N.T.; Pham, N.O.; Nguyen, C.T.; Vu, M.T.; Dinh, V.T.; Le, V.T.; Tran, N.Q. Injectable Nanocurcumin-Dispersed Gelatin–Pluronic Nanocomposite Hydrogel Platform for Burn Wound Treatment. Bull. Mater. Sci. 2019, 42, 71. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Bhubhanil, S.; Talodthaisong, C.; Khongkow, M.; Namdee, K.; Wongchitrat, P.; Yingmema, W.; Hutchison, J.A.; Lapmanee, S.; Kulchat, S. Enhanced Wound Healing Properties of Guar Gum/Curcumin-Stabilized Silver Nanoparticle Hydrogels. Sci. Rep. 2021, 11, 21836. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.H.; Nguyen, T.H.; Tran, H.L.B.; Doan, V.N.; Tran, N.Q. Injectable Nanocurcumin-Formulated Chitosan-g-Pluronic Hydrogel Exhibiting a Great Potential for Burn Treatment. J. Healthc. Eng. 2018, 2018, 5754890. [Google Scholar] [CrossRef]
- Zhou, F.; Song, Z.; Wen, Y.; Xu, H.; Zhu, L.; Feng, R. Transdermal Delivery of Curcumin-Loaded Supramolecular Hydrogels for Dermatitis Treatment. J. Mater. Sci. Mater. Med. 2019, 30, 11. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in Inflammatory Diseases. BioFactors 2013, 39, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and Therapy of Psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Alexander, A.; Singh, M.R.; Singh, D.; Saraf, S.; Saraf, S. Understanding the Prospective of Nano-Formulations towards the Treatment of Psoriasis. Biomed. Pharmacother. 2018, 107, 447–463. [Google Scholar] [CrossRef]
- Filippone, A.; Consoli, G.M.L.; Granata, G.; Casili, G.; Lanza, M.; Ardizzone, A.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Topical Delivery of Curcumin by Choline-Calix[4]Arene-Based Nanohydrogel Improves Its Therapeutic Effect on a Psoriasis Mouse Model. Int. J. Mol. Sci. 2020, 21, 5053. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion Loaded Polymeric Hydrogel for Topical Delivery of Curcumin in Psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Fernández-Romero, A.-M.; Maestrelli, F.; García-Gil, S.; Talero, E.; Mura, P.; Rabasco, A.M.; González-Rodríguez, M.L. Preparation, Characterization and Evaluation of the Anti-Inflammatory Activity of Epichlorohydrin-β-Cyclodextrin/Curcumin Binary Systems Embedded in a Pluronic®/Hyaluronate Hydrogel. Int. J. Mol. Sci. 2021, 22, 13566. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Z.; Wang, L.; Cun, D.; Tong, H.H.Y.; Yan, R.; Chen, X.; Wang, R.; Zheng, Y. Enhanced Topical Penetration, System Exposure and Anti-Psoriasis Activity of Two Particle-Sized, Curcumin-Loaded PLGA Nanoparticles in Hydrogel. J. Control. Release 2017, 254, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The Trends Projection Analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Chivere, V.T.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Nanotechnology-Based Biopolymeric Oral Delivery Platforms for Advanced Cancer Treatment. Cancers 2020, 12, 522. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Y.; Loutfy, S.A.; Kamoun, E.A.; El-Moslamy, S.H.; Radwan, E.M.; Elbehairi, S.E.I. Enhanced Anti-Cancer Activity by Localized Delivery of Curcumin Form PVA/CNCs Hydrogel Membranes: Preparation and in Vitro Bioevaluation. Int. J. Biol. Macromol. 2021, 170, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, L.; Stawny, M.; Mlynarczyk, D.T.; Muszalska-Kolos, I.; Goslinski, T.; Jelińska, A. Role of Curcumin and (−)-Epigallocatechin-3-O-Gallate in Bladder Cancer Treatment: A Review. Cancers 2020, 12, 1801. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Stawny, M.; Piwowarczyk, K.; Mlynarczyk, D.T.; Muszalska-Kolos, I.; Wierzbicka, M.; Goslinski, T.; Jelinska, A. Role of Curcumin in Selected Head and Neck Lesions. Limitations on the Use of the Hep-2 Cell Line: A Critical Review. Biomed. Pharmacother. 2022, 154, 113560. [Google Scholar] [CrossRef]
- Babaei, M.; Davoodi, J.; Dehghan, R.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Thermosensitive Composite Hydrogel Incorporated with Curcumin-Loaded Nanopolymersomes for Prolonged and Localized Treatment of Glioma. J. Drug Deliv. Sci. Technol. 2020, 59, 101885. [Google Scholar] [CrossRef]
- Sadeghi-Abandansari, H.; Pakian, S.; Nabid, M.-R.; Ebrahimi, M.; Rezalotfi, A. Local Co-Delivery of 5-Fluorouracil and Curcumin Using Schiff’s Base Cross-Linked Injectable Hydrogels for Colorectal Cancer Combination Therapy. Eur. Polym. J. 2021, 157, 110646. [Google Scholar] [CrossRef]
- Tan, B.; Wu, Y.; Wu, Y.; Shi, K.; Han, R.; Li, Y.; Qian, Z.; Liao, J. Curcumin-Microsphere/IR820 Hybrid Bifunctional Hydrogels for In Situ Osteosarcoma Chemo- Co -Thermal Therapy and Bone Reconstruction. ACS Appl. Mater. Interfaces 2021, 13, 31542–31553. [Google Scholar] [CrossRef]
- Gao, M.; Xu, H.; Zhang, C.; Liu, K.; Bao, X.; Chu, Q.; He, Y.; Tian, Y. Preparation and Characterization of Curcumin Thermosensitive Hydrogels for Intratumoral Injection Treatment. Drug Dev. Ind. Pharm. 2014, 40, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yue, W.; Ibrahim, K.; Shen, J. A Long-Acting Curcumin Nanoparticle/In Situ Hydrogel Composite for the Treatment of Uveal Melanoma. Pharmaceutics 2021, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Du, L.; Liu, Y.; Li, X.; Li, M.; Jin, Y.; Qian, X. Transdermal Delivery of the in Situ Hydrogels of Curcumin and Its Inclusion Complexes of Hydroxypropyl-β-Cyclodextrin for Melanoma Treatment. Int. J. Pharm. 2014, 469, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Madeo, L.F.; Sarogni, P.; Cirillo, G.; Vittorio, O.; Voliani, V.; Curcio, M.; Shai-Hee, T.; Büchner, B.; Mertig, M.; Hampel, S. Curcumin and Graphene Oxide Incorporated into Alginate Hydrogels as Versatile Devices for the Local Treatment of Squamous Cell Carcinoma. Materials 2022, 15, 1648. [Google Scholar] [CrossRef] [PubMed]
- Shpaisman, N.; Sheihet, L.; Bushman, J.; Winters, J.; Kohn, J. One-Step Synthesis of Biodegradable Curcumin-Derived Hydrogels as Potential Soft Tissue Fillers after Breast Cancer Surgery. Biomacromolecules 2012, 13, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Devkota, H.P.; Adhikari-Devkota, A.; Bhandari, D.R. Curcumin. In Antioxidants Effects in Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 341–352. [Google Scholar] [CrossRef]
- Yu, Q.; Meng, Z.; Liu, Y.; Li, Z.; Sun, X.; Zhao, Z. Photocuring Hyaluronic Acid/Silk Fibroin Hydrogel Containing Curcumin Loaded CHITOSAN Nanoparticles for the Treatment of MG-63 Cells and ME3T3-E1 Cells. Polymers 2021, 13, 2302. [Google Scholar] [CrossRef] [PubMed]
- Teong, B.; Lin, C.-Y.; Chang, S.-J.; Niu, G.C.-C.; Yao, C.-H.; Chen, I.-F.; Kuo, S.-M. Enhanced Anti-Cancer Activity by Curcumin-Loaded Hydrogel Nanoparticle Derived Aggregates on A549 Lung Adenocarcinoma Cells. J. Mater. Sci. Mater. Med. 2015, 26, 49. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Cai, Y.; Zhan, J.; Gao, J.; Song, M.; Shi, Y.; Yang, Z. A Glycyrrhetinic Acid-Modified Curcumin Supramolecular Hydrogel for Liver Tumor Targeting Therapy. Sci. Rep. 2017, 7, 44210. [Google Scholar] [CrossRef]
- Omidi, S.; Pirhayati, M.; Kakanejadifard, A. Co-Delivery of Doxorubicin and Curcumin by a pH-Sensitive, Injectable, and in Situ Hydrogel Composed of Chitosan, Graphene, and Cellulose Nanowhisker. Carbohydr. Polym. 2020, 231, 115745. [Google Scholar] [CrossRef] [PubMed]
- Osorno, L.; Brandley, A.; Maldonado, D.; Yiantsos, A.; Mosley, R.; Byrne, M. Review of Contemporary Self-Assembled Systems for the Controlled Delivery of Therapeutics in Medicine. Nanomaterials 2021, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Altunbas, A.; Lee, S.J.; Rajasekaran, S.A.; Schneider, J.P.; Pochan, D.J. Encapsulation of Curcumin in Self-Assembling Peptide Hydrogels as Injectable Drug Delivery Vehicles. Biomaterials 2011, 32, 5906–5914. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liu, Y.; Qin, J.; Ye, T.; Wang, S. A Novel Pellets/Thermosensitive Hydrogel Depot with Low Burst Release for Long-Term Continuous Drug Release: Preparation, Characterization, in Vitro and in Vivo Studies. J. Drug Deliv. Sci. Technol. 2020, 60, 102050. [Google Scholar] [CrossRef]
- Khan, S.; Minhas, M.U.; Ahmad, M.; Sohail, M. Self-Assembled Supramolecular Thermoreversible β-Cyclodextrin/Ethylene Glycol Injectable Hydrogels with Difunctional Pluronic® 127 as Controlled Delivery Depot of Curcumin. Development, Characterization and in Vitro Evaluation. J. Biomater. Sci. Polym. Ed. 2018, 29, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Akhtar, N.; Minhas, M.U.; Shah, H.; Khan, K.U.; Thakur, R.R.S. A Difunctional Pluronic®127-Based in Situ Formed Injectable Thermogels as Prolonged and Controlled Curcumin Depot, Fabrication, in Vitro Characterization and in Vivo Safety Evaluation. J. Biomater. Sci. Polym. Ed. 2021, 32, 281–319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Dong, X.; Liu, H.; Wang, Y.; Zhang, L.; Sun, Y. Multifunctionality of Self-Assembled Nanogels of Curcumin-Hyaluronic Acid Conjugates on Inhibiting Amyloid β-Protein Fibrillation and Cytotoxicity. React. Funct. Polym. 2016, 104, 22–29. [Google Scholar] [CrossRef]
- Ribeiro, T.D.C.; Sábio, R.M.; Luiz, M.T.; De Souza, L.C.; Fonseca-Santos, B.; Cides Da Silva, L.C.; Fantini, M.C.D.A.; Planeta, C.D.S.; Chorilli, M. Curcumin-Loaded Mesoporous Silica Nanoparticles Dispersed in Thermo-Responsive Hydrogel as Potential Alzheimer Disease Therapy. Pharmaceutics 2022, 14, 1976. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Fang, C.-H.; Yang, C.-Y.; Liang, Y.-J.; Lin, F.-H. Investigating a Curcumin-Loaded PLGA-PEG-PLGA Thermo-Sensitive Hydrogel for the Prevention of Alzheimer’s Disease. Antioxidants 2022, 11, 727. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; Salman, A.S.; Ibrahim, M.I.M.; El-Kady, A.A.; Abdel-Aziem, S.H.; Hassan, N.S.; Waly, A.I. Curcumin Nanoparticles Loaded Hydrogels Protects against Aflatoxin B1-Induced Genotoxicity in Rat Liver. Food Chem. Toxicol. 2016, 94, 159–171. [Google Scholar] [CrossRef]
- Namdari, M.; Eatemadi, A. Cardioprotective Effects of Curcumin-Loaded Magnetic Hydrogel Nanocomposite (Nanocurcumin) against Doxorubicin-Induced Cardiac Toxicity in Rat Cardiomyocyte Cell Lines. Artif. Cells Nanomed. Biotechnol. 2017, 45, 731–739. [Google Scholar] [CrossRef]
- Mohandas, A.; Rangasamy, J. Nanocurcumin and Arginine Entrapped Injectable Chitosan Hydrogel for Restoration of Hypoxia Induced Endothelial Dysfunction. Int. J. Biol. Macromol. 2021, 166, 471–482. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, E.Y.; Shin, M.E.; Choi, M.J.; Carlomagno, C.; Song, J.E.; Khang, G. Enhanced Retinal Pigment Epithelium (RPE) Regeneration Using Curcumin/Alginate Hydrogels: In Vitro Evaluation. Int. J. Biol. Macromol. 2018, 117, 546–552. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Shang, L.; Mao, Y. GelMA Hydrogel Scaffold Containing Curcumin-Loaded Solid Lipid Nanoparticles Promotes the Regeneration of Degenerative Discs. SN Appl. Sci. 2023, 5, 243. [Google Scholar] [CrossRef]
- Sun, Q.; Yin, W.; Ru, X.; Liu, C.; Song, B.; Qian, Z. Dual Role of Injectable Curcumin-Loaded Microgels for Efficient Repair of Osteoarthritic Cartilage Injury. Front. Bioeng. Biotechnol. 2022, 10, 994816. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-C.; Yoon, S.J.; Noh, K.; Lee, D.-W. Dual Effect of Curcumin/BMP-2 Loaded in HA/PLL Hydrogels on Osteogenesis In Vitro and In Vivo. J. Nanosci. Nanotechnol. 2017, 17, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shi, X.; Li, L.; Tan, Z.; Feng, F.; Li, J.; Pang, M.; Wang, X.; He, L. An Injectable and Self-Healing Hydrogel with Controlled Release of Curcumin to Repair Spinal Cord Injury. Bioact. Mater. 2021, 6, 4816–4829. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Sheriffa Begum, K.M.M.; Arthanareeswaran, G. Biomass-Derived Dialdehyde Cellulose Cross-Linked Chitosan-Based Nanocomposite Hydrogel with Phytosynthesized Zinc Oxide Nanoparticles for Enhanced Curcumin Delivery and Bioactivity. J. Agric. Food Chem. 2019, 67, 10880–10890. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gong, X.; Guo, X.; Liu, C.; Fan, Y.-Y.; Zhang, J.; Niu, B.; Li, W. Characterization, Release, and Antioxidant Activity of Curcumin-Loaded Sodium Alginate/ZnO Hydrogel Beads. Int. J. Biol. Macromol. 2019, 121, 1118–1125. [Google Scholar] [CrossRef]
- Heikal, E.J.; Kaoud, R.M.; Gad, S.; Mokhtar, H.I.; Aldahish, A.A.; Alzlaiq, W.A.; Zaitone, S.A.; Moustafa, Y.M.; Hammady, T.M. Design and Optimization of Omeprazole-Curcumin-Loaded Hydrogel Beads Coated with Chitosan for Treating Peptic Ulcers. Pharmaceuticals 2023, 16, 795. [Google Scholar] [CrossRef]
- Naderi, Z.; Azizian, J. Synthesis and Characterization of Carboxymethyl Chitosan/Fe3O4 and MnFe2O4 Nanocomposites Hydrogels for Loading and Release of Curcumin. J. Photochem. Photobiol. B Biol. 2018, 185, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, D.B.E.D.; Marzok, S. In Situ Supersaturable Polyhydrogels: A Feasible Modification of the Conventional Hydrogels for the Enhanced Delivery of Stomach Specific Hydrophobic Drugs. J. Drug Deliv. Sci. Technol. 2020, 58, 101744. [Google Scholar] [CrossRef]
- Huang, S.; Wang, J.; Shang, Q. Development and Evaluation of a Novel Polymeric Hydrogel of Sucrose Acrylate-Co-Polymethylacrylic Acid for Oral Curcumin Delivery. J. Biomater. Sci. Polym. Ed. 2017, 28, 194–206. [Google Scholar] [CrossRef] [PubMed]






| Type of Curcumin–Hydrogel | Main Advantage | References |
|---|---|---|
| Nano-based oral delivery systems | Controlled/prolonged release | [30,32] |
| Nano-based topical delivery systems | Better skin permeability | [31,33] |
| Injectable delivery systems | Controlled release | [34,35] |
| Histo- Logical Score | ZO-1 Expression | Occludin Expression | Mast Cell Proliferation | TNF-α | IL-1β | iNOS Levels | |
|---|---|---|---|---|---|---|---|
| [% of Total Tissue Count] | [Number/mm2] | [% of Total Tissue Count] | |||||
| Control | ND | ~7% | ~7% | ~0 | ~0% | ~0% | ~0% |
| Imiquimod | ~4 | ~2% | ~1.5% | ~50 | ~7% | ~7.5% | ~7% |
| Imiquimod + hydrogel | ~1 | ~5% | ~5% | ~10 | ~2% | ~2% | ~2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachowiak, M.; Mlynarczyk, D.T.; Dlugaszewska, J. Wondrous Yellow Molecule: Are Hydrogels a Successful Strategy to Overcome the Limitations of Curcumin? Molecules 2024, 29, 1757. https://doi.org/10.3390/molecules29081757
Stachowiak M, Mlynarczyk DT, Dlugaszewska J. Wondrous Yellow Molecule: Are Hydrogels a Successful Strategy to Overcome the Limitations of Curcumin? Molecules. 2024; 29(8):1757. https://doi.org/10.3390/molecules29081757
Chicago/Turabian StyleStachowiak, Magdalena, Dariusz T. Mlynarczyk, and Jolanta Dlugaszewska. 2024. "Wondrous Yellow Molecule: Are Hydrogels a Successful Strategy to Overcome the Limitations of Curcumin?" Molecules 29, no. 8: 1757. https://doi.org/10.3390/molecules29081757
APA StyleStachowiak, M., Mlynarczyk, D. T., & Dlugaszewska, J. (2024). Wondrous Yellow Molecule: Are Hydrogels a Successful Strategy to Overcome the Limitations of Curcumin? Molecules, 29(8), 1757. https://doi.org/10.3390/molecules29081757