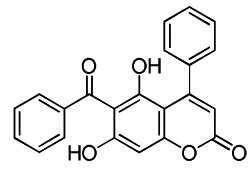
Anti-UVC Irradiation and Metal Chelation Properties of 6-Benzoyl-5,7-dihydroxy-4-phenyl-chromen-2-one: An Implications for Anti-Cataract Agent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Results
2.2.1. Turbidity Assay of γ-Crystallin and Comparison of Anti-UVC Activity
2.2.2. Evaluation of the Substitution Groups
2.2.3. Protection of DNA
2.2.4. Compound 7 Protects Porcine Lens Proteins from Oxidative Damage by Hydroxyl Radicals
2.3. Iron-Chelating Activity
3. Experimental Section
3.1. General Experimental Conditions
3.2. Preparation of Coumarin Derivatives 3–11
3.3. Iron-Chelating Activity
3.4. 1H-NMR Studies of Metal Chelation
3.5. Absorption Spectra of Coumarin Derivatives
3.6. Biological Assay
3.6.1. Purification of Porcine γ-Crystallin
3.6.2. Turbidity Assays of γ-Crystallin under UVC Irradiation
3.6.3. Protection of Supercoiled DNA from Strand Breakage by UVC Irradiation
3.6.4. Fenton Reaction
4. Conclusions
Acknowledgments
References
- Davies, MJ; Truscott, RJ. Photo-oxidation of proteins and its role in cataractogenesis. J. Photochem. Photobiol. B 2001, 63, 114–125. [Google Scholar]
- Brian, G; Taylor, H. Cataract blindness—Challenges for the 21st century. Bull. World Health Organ 2001, 79, 249–256. [Google Scholar]
- Lodovici, M; Caldini, S; Morbidelli, L; Akpan, V; Ziche, M; Dolara, P. Protective effect of 4-coumaric acid from UVB ray damage in the rabbit eye. Toxicology 2009, 255, 1–5. [Google Scholar]
- Hoult, JR; Paya, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol 1996, 27, 713–722. [Google Scholar]
- Fylaktakidou, KC; Hadjipavlou-Litina, DJ; Litinas, KE; Nicolaides, DN. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des 2004, 10, 3813–3833. [Google Scholar]
- Borges, F; Roleira, F; Milhazes, N; Santana, L; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem 2005, 12, 887–916. [Google Scholar]
- Musicki, B; Periers, AM; Laurin, P; Ferroud, D; Benedetti, Y; Lachaud, S; Chatreaux, F; Haesslein, JL; Iltis, A; Pierre, C; et al. Improved antibacterial activities of coumarin antibiotics bearing 5,5′-dialkylnoviose: Biological activity of RU79115. Bioorg. Med. Chem. Lett 2000, 10, 1695–1699. [Google Scholar]
- Thaisrivongs, S; Watenpaugh, KD; Howe, WJ; Tomich, PK; Dolak, LA; Chong, KT; Tomich, CC; Tomasselli, AG; Turner, SR; Strohbach, JW; et al. Structure-based design of novel HIV protease inhibitors: Carboxamide-containing 4-hydroxycoumarins and 4-hydroxy-2-pyrones as potent nonpeptidic inhibitors. J. Med. Chem 1995, 38, 3624–3637. [Google Scholar]
- Valenti, P; Fabbri, G; Rampa, A; Bisi, A; Gobbi, S; Da Re, P; Carrara, M; Sgevano, A; Cima, L. Synthesis and antitumor activity of some analogues of flavone acetic acid. Anticancer Drug Des 1996, 11, 243–252. [Google Scholar]
- Stirling, Y. Warfarin-induced changes in procoagulant and anticoagulant proteins. Blood Coagul. Fibrinolysis 1995, 6, 361–373. [Google Scholar]
- Pedersen, JZ; Oliveira, C; Incerpi, S; Kumar, V; Fiore, AM; De Vito, P; Prasad, AK; Malhotra, SV; Parmar, VS; Saso, L. Antioxidant activity of 4-methylcoumarins. J. Pharm. Pharmacol 2007, 59, 1721–1728. [Google Scholar]
- Manojkumar, P; Ravi, TK; Gopalakrishnan, S. Antioxidant and antibacterial studies of arylazopyrazoles and arylhydrazonopyrazolones containing coumarin moiety. Eur. J. Med. Chem 2009, 44, 4690–4694. [Google Scholar]
- Thuong, PT; Hung, TM; Ngoc, TM; Ha do, T; Min, BS; Kwack, SJ; Kang, TS; Choi, JS; Bae, K. Antioxidant activities of coumarins from Korean medicinal plants and their structure-activity relationships. Phytother. Res 2009, 24, 101–106. [Google Scholar]
- Manojkumar, P; Ravi, TK; Subbuchettiar, G. Synthesis of coumarin heterocyclic derivatives with antioxidant activity and in vitro cytotoxic activity against tumour cells. Acta Pharm 2009, 59, 159–170. [Google Scholar]
- Gacche, RN; Gond, DS; Dhole, NA; Dawane, BS. Coumarin Schiff-bases: As antioxidant and possibly anti-inflammatory agents. J. Enzym. Inhib. Med. Chem 2006, 21, 157–161. [Google Scholar]
- Torres, R; Faini, F; Modak, B; Urbina, F; Labbe, C; Guerrero, J. Antioxidant activity of coumarins and flavonols from the resinous exudate of Haplopappus multifolius. Phytochemistry 2006, 67, 984–987. [Google Scholar]
- Kontogiorgis, CA; Savvoglou, K; Hadjipavlou-Litina, DJ. Antiinflammatory and antioxidant evaluation of novel coumarin derivatives. J. Enzym. Inhib. Med. Chem 2006, 21, 21–29. [Google Scholar]
- Yu, J; Wang, L; Walzem, RL; Miller, EG; Pike, LM; Patil, BS. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J. Agric. Food. Chem 2005, 53, 2009–2014. [Google Scholar]
- Lee, BC; Lee, SY; Lee, HJ; Sim, GS; Kim, JH; Kim, JH; Cho, YH; Lee, DH; Pyo, HB; Choe, TB; Moon, DC; Yun, YP; Hong, JT. Anti-oxidative and photo-protective effects of coumarins isolated from Fraxinus chinensis. Arch. Pharm. Res 2007, 30, 1293–1301. [Google Scholar]
- Lin, CM; Huang, ST; Lee, FW; Kuo, HS; Lin, MH. 6-Acyl-4-aryl/alkyl-5,7-dihydroxycoumarins as anti-inflammatory agents. Bioorg. Med. Chem 2006, 14, 4402–4409. [Google Scholar]
- Kostova, I. Synthetic and natural coumarins as antioxidants. Mini Rev. Med. Chem 2006, 6, 365–374. [Google Scholar]
- Kennedy, M; Kim, KH; Harten, B; Brown, J; Planck, S; Meshul, C; Edelhauser, H; Rosenbaum, JT; Armstrong, CA; Ansel, JC. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest. Ophthalmol. Vis. Sci 1997, 38, 2483–2491. [Google Scholar]
- Choy, CK; Cho, P; Chung, WY; Benzie, IF. Water-soluble antioxidants in human tears: Effect of the collection method. Invest. Ophthalmol. Vis. Sci 2001, 42, 3130–3134. [Google Scholar]
- Chandra, P; Hegde, KR; Varma, SD. Possibility of topical antioxidant treatment of cataracts: Corneal penetration of pyruvate in humans. Ophthalmologica 2009, 223, 136–138. [Google Scholar]
- Kawada, H; Kojima, M; Kimura, T; Natori, S; Sasaki, K; Sasaki, H. Effect of 5-S-GAD on UV-B-induced cataracts in rats. Jpn. J. Ophthalmol 2009, 53, 531–535. [Google Scholar]
- Varma, SD; Hegde, KR; Kovtun, S. UV-B-induced damage to the lens in vitro: Prevention by caffeine. J. Ocul. Pharmacol. Ther 2008, 24, 439–444. [Google Scholar]
- Lee, JS; Samejima, T; Liao, JH; Wu, SH; Chiou, SH. Physiological role of the association complexes of alpha-crystallin and its substrates on the chaperone activity. Biochem. Biophys. Res. Commun 1998, 244, 379–383. [Google Scholar]
- Liao, JH; Lee, JS; Chiou, SH. Distinct roles of alphaA- and alphaB-crystallins under thermal and UV stresses. Biochem. Biophys. Res. Commun 2002, 295, 854–861. [Google Scholar]
- Sawada, H; Fukuchi, T; Abe, H. Oxidative stress markers in aqueous humor of patients with senile cataracts. Curr. Eye Res 2009, 34, 36–41. [Google Scholar]
- Zoric, L; Kosanovic-Jakovic, N; Colak, E; Radosavljevic, A; Jaksic, V; Stevic, S. Oxidative stress in association with risk factors for the occurrence and development of age-related macular degeneration. Vojnosanit. Pregl 2008, 65, 313–318. [Google Scholar]
- Berthoud, VM; Beyer, EC. Oxidative stress, lens gap junctions, and cataracts. Antioxid. Redox. Signal 2009, 11, 339–353. [Google Scholar]
- Li, L; Duker, JS; Yoshida, Y; Niki, E; Rasmussen, H; Russell, RM; Yeum, K-J. Oxidative stress and antioxidant status in older adults with early cataract. Eye (Lond. ) 2009, 23, 1464–1468. [Google Scholar]
- Gul, A; Rahman, MA; Hasnain, SN; Salim, A; Simjee, SU. Could oxidative stress associate with age products in cataractogenesis? Curr. Eye Res 2008, 33, 669–675. [Google Scholar]
- Williams, DL. Oxidative stress and the eye. Vet. Clin. North Am. Small Anim. Pract 2008, 38, 179–192. [Google Scholar]
- Murray, RD. Coumarins. Nat. Prod. Rep 1989, 6, 591–624. [Google Scholar]
- Chin, YP; Huang, WJ; Hsu, FL; Lin, YL; Lin, MH. Synthesis and evaluation of antibacterial activities of 5,7-Dihydroxycoumarin derivatives. Arch. Pharm 2011, 344, 386–393. [Google Scholar]
- Graw, J. Mouse models of cataract. J. Genet 2009, 88, 469–486. [Google Scholar]
- Wang, Y; Petty, S; Trojanowski, A; Knee, K; Goulet, D; Mukerji, I; King, J. Formation of amyloid fibrils in vitro from partially unfolded intermediates of human gammaC-crystallin. Invest. Ophthalmol. Vis. Sci 2010, 51, 672–678. [Google Scholar]
- Skouri-Panet, F; Bonnete, F; Prat, K; Bateman, OA; Lubsen, NH; Tardieu, A. Lens crystallins and oxidation: The special case of gammas. Biophys. Chem 2001, 89, 65–76. [Google Scholar]
- Azrague, K; Bonnefille, E; Pradines, V; Pimienta, V; Oliveros, E; Maurette, MT; Benoit-Marquié, F. Hydrogen peroxide evolution during V-UV photolysis of water. Photochem. Photobiol. Sci 2005, 4, 406–408. [Google Scholar]
- Zhan, M. Determination of photochemically-generated reactive oxygen species in natural water. J. Environ. Sci. (China) 2009, 21, 303–306. [Google Scholar]
- Wagner, AE; Huebbe, P; Konishi, T; Rahman, MM; Nakahara, M; Matsugo, S; Rimbach, G. Free radical scavenging and antioxidant activity of ascorbigen versus ascorbic acid: Studies in vitro and in cultured human keratinocytes. J. Agric. Food. Chem 2008, 56, 11694–11699. [Google Scholar]
- Warren, JJ; Mayer, JM. Surprisingly long-lived ascorbyl radicals in acetonitrile: Concerted proton-electron transfer reactions and thermochemistry. J. Am. Chem. Soc 2008, 130, 7546–7547. [Google Scholar]
- Oriowo, OM; Cullen, AP; Chou, BR; Sivak, JG. Action spectrum and recovery for in vitro UV-induced cataract using whole lenses. Invest. Ophthalmol. Vis. Sci 2001, 42, 2596–2602. [Google Scholar]
- Wu, TH; Liao, JH; Hou, WC; Huang, FY; Maher, TJ; Hu, CC. Astaxanthin protects against oxidative stress and calcium-induced porcine lens protein degradation. J. Agric. Food. Chem 2006, 54, 2418–2423. [Google Scholar]
- Lo, LC; Chu, CY. Development of highly selective and sensitive probes for hydrogen peroxide. Chem. Commun 2003, 2003, 2728–2729. [Google Scholar]
- Lewis, S; Lynch, A; Bachas, L; Hampson, S; Ormsbee, L; Bhattacharyya, D. Chelate-modified fenton reaction for the degradation of trichloroethylene in aqueous and two-phase systems. Environ. Eng. Sci 2009, 26, 849–859. [Google Scholar]
- Perez, CA; Wei, Y; Guo, M. Iron-binding and anti-Fenton properties of baicalein and baicalin. J. Inorg. Biochem 2009, 103, 326–332. [Google Scholar]
- Jurd, L; Corse, J; King, AD, Jr; Bayne, H; Mihara, K. Phytochemistry Antimicrobial properties of 6,7-dihydroxy-7,8-dihydroxy-,6-hydroxy- and 8-hydroxycoumarins. Phytochemistry 1971, 10, 2971–2974. [Google Scholar]
- Shi, Z; He, C. Efficient functionalization of aromatic C–H bonds catalyzed by gold(III) under mild and solvent-free conditions. J. Org. Chem 2004, 69, 3669–3671. [Google Scholar]
- Lopes, GK; Schulman, HM; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim. Biophys. Acta 1999, 1472, 142–152. [Google Scholar]
- Wu, TH; Liao, JH; Hsu, FL; Wu, HR; Shen, CK; Yuann, JMP; Chen, ST. Grape Seed Proanthocyanidin Extract Chelates Iron and Attenuates the Toxic Effects of 6-Hydroxydopamine: Implications for Parkinsons’s Disease. J. Food Biochem 2010, 34, 244–262. [Google Scholar]
- Liao, JH; Hung, CC; Lee, JS; Wu, SH; Chiou, SH. Characterization, cloning, and expression of porcine alpha B crystallin. Biochem. Biophys. Res. Commun 1998, 244, 131–137. [Google Scholar]
- Keenan, J; Orr, DF; Pierscionek, BK. Patterns of crystallin distribution in porcine eye lenses. Mol. Vis 2008, 14, 1245–1253. [Google Scholar]
- Laemmli, UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Huang, FY; Chia, CM; Ho, Y. The formation of oxidatively induced high-molecular-weight aggregate of alpha-/gamma-crystallins. Biochem. Biophys. Res. Commun 1999, 260, 60–65. [Google Scholar]
- Wood, AM; Truscott, RJ. UV filters in human lenses: Tryptophan catabolism. Exp. Eye Res 1993, 56, 317–325. [Google Scholar]
- Wood, AM; Truscott, RJ. Ultraviolet filter compounds in human lenses: 3-hydroxykynurenine glucoside formation. Vis. Res 1994, 34, 1369–1374. [Google Scholar]
- Truscott, RJ; Wood, AM; Carver, JA; Sheil, MM; Stutchbury, GM; Zhu, J; Kilby, GW. A new UV-filter compound in human lenses. FEBS Lett 1994, 348, 173–176. [Google Scholar]
- Loh, A; Hadziahmetovic, M; Dunaief, JL. Iron homeostasis and eye disease. Biochim. Biophys. Acta 2009, 1790, 637–649. [Google Scholar]
- Goralska, M; Nagar, S; Colitz, CM; Fleisher, LN; McGahan, MC. Changes in ferritin H- and L-chains in canine lenses with age-related nuclear cataract. Invest. Ophthalmol. Vis. Sci 2009, 50, 305–310. [Google Scholar]
- Sneed, SR; Weingeist, TA. Management of siderosis bulbi due to a retained iron-containing intraocular foreign body. Ophthalmology 1990, 97, 375–379. [Google Scholar]
- Hope-Ross, M; Mahon, GJ; Johnston, PB. Ocular siderosis. Eye (Lond. ) 1993, 7, 419–425. [Google Scholar]
- Girelli, D; Corrocher, R; Bisceglia, L; Olivieri, O; de Franceschi, L; Zelante, L; Gasparini, P. Molecular basis for the recently described hereditary hyperferritinemia-cataract syndrome: A mutation in the iron-responsive element of ferritin l-subunit gene (the “Verona mutation”). Blood 1995, 86, 4050–4053. [Google Scholar]








| cpd no. | 2 | 3 | 4 | 5 | 6 |
| OD | 0.636 ± 0.041 | 0.193 ± 0.043 | −0.031 ± 0.008 | 0.698 ± 0.150 | 0.0003 ± 0.074 |
| cpd no. | 7 | 8 | 9 | 10 | 11 |
| OD | −0.041 ± 0.012 | 0.502 ± 0.012 | 0.284 ± 0.035 | 0.364 ± 0.018 | 0.406 ± 0.032 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liao, J.-H.; Wu, T.-H.; Hsu, F.-L.; Huang, Y.-S.; Chiang, P.-H.; Huang, Z.-Y.; Huang, C.-H.; Wu, S.-H.; Lin, M.-H. Anti-UVC Irradiation and Metal Chelation Properties of 6-Benzoyl-5,7-dihydroxy-4-phenyl-chromen-2-one: An Implications for Anti-Cataract Agent. Int. J. Mol. Sci. 2011, 12, 7059-7076. https://doi.org/10.3390/ijms12107059
Liao J-H, Wu T-H, Hsu F-L, Huang Y-S, Chiang P-H, Huang Z-Y, Huang C-H, Wu S-H, Lin M-H. Anti-UVC Irradiation and Metal Chelation Properties of 6-Benzoyl-5,7-dihydroxy-4-phenyl-chromen-2-one: An Implications for Anti-Cataract Agent. International Journal of Molecular Sciences. 2011; 12(10):7059-7076. https://doi.org/10.3390/ijms12107059
Chicago/Turabian StyleLiao, Jiahn-Haur, Tzu-Hua Wu, Feng-Lin Hsu, Yi-Shiang Huang, Po-Hung Chiang, Zih-You Huang, Chi-Hsien Huang, Shih-Hsiung Wu, and Mei-Hsiang Lin. 2011. "Anti-UVC Irradiation and Metal Chelation Properties of 6-Benzoyl-5,7-dihydroxy-4-phenyl-chromen-2-one: An Implications for Anti-Cataract Agent" International Journal of Molecular Sciences 12, no. 10: 7059-7076. https://doi.org/10.3390/ijms12107059
APA StyleLiao, J. -H., Wu, T. -H., Hsu, F. -L., Huang, Y. -S., Chiang, P. -H., Huang, Z. -Y., Huang, C. -H., Wu, S. -H., & Lin, M. -H. (2011). Anti-UVC Irradiation and Metal Chelation Properties of 6-Benzoyl-5,7-dihydroxy-4-phenyl-chromen-2-one: An Implications for Anti-Cataract Agent. International Journal of Molecular Sciences, 12(10), 7059-7076. https://doi.org/10.3390/ijms12107059