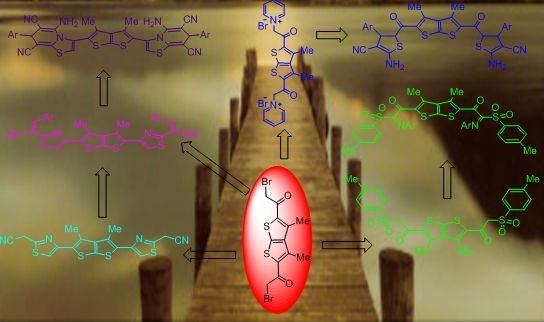
A Novel and Expedient Approach to New Thiazoles, Thiazolo[3,2-a]pyridines, Dihydrothiophenes, and Hydrazones Incorporating Thieno[2,3-b]thiophene Moiety
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. 2,2′-(4,4′-(3,4′-Dimethylthieno[2,3-b]thiophene-2,5-diyl)Bis(thiazole-4,2 diyl))diacetonitrile (2)
3.2. General Procedure for the Synthesis of Compounds 3a-c (GP1)
3.2.1. 4,4′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(thiazole-4,2-Diyl))bis (3-aryle acrylonitrile) (3a–c)
3.2.2. 2,2′-(4,4′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5- diyl)bis(triazole -4,2-diyl))bis (3-phenylacrylonitrile) (3a)
3.2.3. 2,2′-(4,4′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(thiazole-4,2-Diyl))bis (3-(4-chlorophenyl)acrylonitrile (3b)
3.2.4. 2,2′-(4,4′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(thiazole-4,2-diyl))bis (3-(4-methoxyphenyl)acrylonitrile (3c)
3.3. General Procedure for the Synthesis of Compounds 4a-c (GP2)
3.3.1. 3,3′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(5-amino-7-aryle-7H-thiazolo[3,2-a]pyridine-6,8-dicarbonitrile) (4a-c)
3.3.2. 3,3′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(5-amino-7-phenyl-7H-thiazolo [3,2-a]pyridine-6,8-dicarbonitrile (4a)
3.3.3. 3,3′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(5-amino-7-(4-chlorophenyl)-7Hthiazolo[ 3,2-a]pyridine-6,8-dicarbonitrile (4b)
3.3.4. 3,3′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(5-amino-7-(4-methoxyphenyl)-7Hthiazolo[ 3,2-a]pyridine-6,8-dicarbonitrile) (4c)
3.4. 1,1′-(2,2′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-diyl)bis(2-oxoethane-2,1-diyl))dipyridinium Bromide (5)
3.5. General Procedure for the Synthesis of Compounds 6a-d (GP3)
3.5.1. 5,5′-(3,4-Dimethylthieno[2,3-b]thiophene-2,5-dicarbonyl)bis(2-amino-4-aryl-4, 5-dihydrothiophene-3-carbonitrile) (6a-d)
3.5.2. 4,4′-5,5′(3,4-Dimethylthieno[2,3-b]thiophene-2,5-dicarbonyl)bis(2-amino-4-phenyl-4, 5-dihydrothiphene-3-corbonitrile (6a)
3.5.3. 4,4′-5,5′(3,4-Dimethylthieno[2,3-b]thiophene-2,5-dicarbonyl)bis(2-amino-4-(4-chlorophenyl)-4,5-dihydrothiphene-3-corbonitrile (6b)
3.5.4. 4,4′-5,5′(3,4-Dimethylthieno[2,3-b]thiophene-2,5-dicarbonyl)bis(2-amino-4-(4-hydroxyphenyl)-4,5-dihydrothiphene-3-corbonitrile (6c)
3.5.5. 4,4′-5,5′(3,4-Dimethylthieno[2,3-b]thiophene-2,5-dicarbonyl)bis(2-amino-4-(4-methoxyphenyl)-4,5-dihydrothiphene-3-corbonitrile (6d)
3.6. 1,1′-(3,4-Dimethyl-5-(2-tosylacetyl)thieno[2,3-b]thiophen-2-yl)-2-tosylethanone (9)
3.7. General Procedure for the Synthesis of Compounds 10a-c (GP4)
3.7.1. 2-(2-Arylhydrazono)-1-(5-((Z)-2-(2-chlorohydrazono)-2-tosylacetyl)-3,4-dimethylthieno [2,3-b]thiophen-2-yl)-2-tosylethanone (10a–c)
3.7.2. 1-(3,4-Dimethyl-5-2-(2-phenylhydrazono)-2-tosylacetyl)thieno[2,3-b]thiophen-2-yl)-2-(2- phenylhydrazono)-2-tosylethanone (10a)
3.7.3. 2-(2-(4-Chlorophenyl) hydrazono)-1-(5-2-(2-(4-chlorophenyl) hydrazono)-2-tosylacetyl)-3, 4-dimethylthieno[2,3-b]thiophen-2-yl)-2-tosylethanone (10b)
3.7.4. 1-(3,4-Dimethyl-5-2-(2-(p-tolyl)hydrazono)-2-tosylacetyl)thieno[2,3-b]thiophen-2-yl)-2-(2- (p-tolyl)hydrazono)-2-tosylethanone (10c)
4. Conclusions
Acknowledgments
References
- Jarak, I.; Kralj, M.; Piantanida, I.; Suman, L.; Zinic, M.; Pavelic, K.; Karminski-Zamola, G. Novel cyano- and amidino-substituted derivatives of thieno[2,3-b]- and thien-o[3,2-b]thiophene-2-carboxanilides and thieno[30,20:4,5]thieno- and thieno[20,30:4,5]thieno[2,3-c]quinolones: Synthesis, photochemical synthesis, DNA binding, and antitumor evaluation. Bioorg. Med. Chem 2006, 14, 2859–2868. [Google Scholar]
- Peters, D.; Hornfeldt, A.B.; Gronowitz, S. Synthesis of various 5-substituted uracils. J. Heterocycl. Chem 1990, 27, 2165–2173. [Google Scholar]
- Kukolja, S.; Draheim, S.E.; Graves, B.J.; Hunden, D.C.; Pfeil, J.L.; Cooper, R.D.G.; Ott, J.L.; Couter, F.T. Orally absorbable cephalosporin antibiotics. 2. Structure-activity studies of bicyclic glycine derivatives of 7-aminodeacetoxycephalosporanic acid. J. Med. Chem 1985, 28, 1896–1903. [Google Scholar]
- Prugh, J.D.; Hartman, G.D.; Mallorga, P.J.; McKeever, B.M.; Michelson, S.R.; Murcko, M.A.; Schwam, H.; Smith, R.L; Sondey, J.M.; Springer, J.P.; et al. 5-substituted thieno[2,3-b]thiophene-2-sulfonamides. J. Med. Chem 1991, 34, 1805–1818. [Google Scholar]
- Hartman, G.D.; Ansdale, P.A. Substituted thieno[2,3-b]thiophene-2-sulfonamides as antiglaucoma agents. U.S. Patent 4,806,562, 21 February 1989. [Google Scholar]
- Mabkhot, N.Y.; Barakat, A.; Al-Majid, A.M.; Al-Othman, Z.A.; Alamary, A.S. A facile and convenient synthesis of some novel hydrazones, schiff’s base and pyrazoles incorporating thieno[2,3-b]thiophenes. Int. J. Mol. Sci 2011, 12, 7824–7834. [Google Scholar]
- Mabkhot, Y.N.; Kheder, N.A.; Al-Majid, A.M. Facile and convenient synthesis of new thieno[2,3-b]thiophene derivatives. Molecules 2010, 15, 9418–9426. [Google Scholar]
- Mabkhot, Y.N. synthesis and chemical characterisation of new bis-thieno[2,3-b]thiophene derivatives. Molecules 2010, 15, 3329–3337. [Google Scholar]
- Mabkhot, Y.N. Synthesis and analysis of some bis-heterocyclic compounds containing Sulphur. Molecules 2009, 14, 1904–1914. [Google Scholar]
- Mabkhot, Y.N.; Al-Majid, A.M.; Barakat, A.; Alshahrani, S.; Siddiqui, Y. 1,1′-(3-Methyl-4-phenylthieno[2,3-b]thiophene-2,5-diyl)diethanone as building block in heterocyclic synthesis. Novel synthesis of some pyrazoles, and pyrimidines derivatives. Molecules 2011, 16, 6502–6511. [Google Scholar]
- Mabkhot, Y.N.; Al-Majid, A.M.; Alamary, A.S. Synthesis and chemical characterisation of some new diheteroaryl thienothiophene derivatives. Molecules 2011, 16, 7706–7714. [Google Scholar]
- Mabkhot, N.Y.; Barakat, A.; Al-Majid, A.M.; Alshahrani, S.A. Comprehensive and facile synthesis of some functionalized bis-heterocyclic compounds containing a thieno[2,3-b]thiophene motif. Int. J. Mol. Sci 2012, 13, 2263–2275. [Google Scholar]
- Mabkhoot, Y.N. Synthesis and analysis of some bis-heterocyclic compounds containing sulphur. Molecules 2009, 14, 1904–1914. [Google Scholar]
- Eicher, T.; Hauptmann, S.; Speicher, A. Five-Membered Heterocycle. In The Chemistry of Heterocycles; Wiley-VCH: New York, NY, USA, 2003. [Google Scholar]
- Gronowitz, S. The Chemistry of Heterocyclic Compounds: Thiophene and Its Derivatives; Gronowitz, S., Ed.; Wiley: New York, NY, USA, 1991; Volume 44, Part 3, Chapter 2. [Google Scholar]
- Quiroga, J.; Hernandez, P.; Insuasty, B.; Abonia, R.; Cobo, J.; Sanchez, A.; Nogueras, M.; Low, J.N. Control of the reaction between 2-aminobenzothiazoles and mannich bases: Synthesis of pyrido[2,1-b][1,3]benzothiazoles versus [1,3]benzothiazolo[2,3-b]quinazolines. J. Chem. Soc. Perkin Trans. 1 2002, 4, 555–559. [Google Scholar]
- Hutchinson, I.; Jennings, S.A.; Vishnuvajjala, B.R.; Westwell, A.D.; Stevens, M.F.G. Antitumor benzothiazoles: 16 synthesis and pharmaceutical properties of antitumor 2-(4-aminophenyl)benzothiazole amino acid prodrugs. J. Med. Chem 2002, 45, 744–747. [Google Scholar]
- Hargrave, K.D.; Hess, F.K.; Oliver, J.T. N-(4-Substituted-thiazolyl)oxamic acid derivatives, new series of potent, orally active antiallergy agents. J. Med. Chem 1983, 26, 1158–1163. [Google Scholar]
- Patt, W.C.; Hamilton, H.W.; Taylor, M.D.; Ryan, M.J.; Taylor, D.G., Jr; Connolly, C.J.C.; Doherty, A.M.; Klutchko, S.R.; Sircar, I; Steinbaugh, B.A.; et al. Structure-activity relationships of a series of 2-amino-4-thiazole containing renin inhibitors. J. Med. Chem. 1992, 35, 2562–2572. [Google Scholar]
- Sharma, R.N.; Xavier, F.P.; Vasu, K.K.; Chaturvedi, S.C.; Pancholi, S.S. Synthesis of 4-benzyl-1,3-thiazole derivatives as potential anti-inflammatory agents: An analogue-based drug design approach. J. Enzym. Inhib. Med. Chem 2009, 24, 890–897. [Google Scholar]
- Jaen, J.C.; Wise, L.D.; Caprathe, B.W.; Tecle, H; Bergmeier, S.; Humblet, C.C.; Heffner, T.G.; Meltzner, L.T.; Pugsley, T.A. 4-(1,2,5,6-Tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: A novel class of compounds with central dopamine agonist properties. J. Med. Chem 1990, 33, 311–317. [Google Scholar]
- Tsuji, K.; Ishikawa, H. Synthesis and anti-pseudomonal activity of new 2-isocephems with a dihydroxypyridone moiety at C-7. Bioorg. Med. Chem. Lett 1994, 4, 1601–1606. [Google Scholar]
- Bell, F.W.; Cantrell, A.S.; Hogberg, M.; Jaskunas, S.R.; Johansson, N.G.; Jordon, C.L.; Kinnick, M.D.; Lind, P.; Morin, J.M., Jr; Noreen, R.; et al. Phenethylthiazolethiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors. 1: Synthesis and basic structure-activity relationship studies of PETT analogs. J. Med. Chem 1995, 38, 4929–4936. [Google Scholar]
- Lin, R.; Johnson, S.G.; Connolly, P.J.; Wetter, S.K.; Binnun, E.; Hughes, T.V.; Murray, W.V.; Pandey, N.B.; Moreno-Mazza, S.J.; Adams, M.; et al. Synthesis and evaluation of 2,7-diamino-thiazolo[4,5-d] pyrimidine analogues as anti-tumor epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett 2009, 19, 2333–2337. [Google Scholar]
- Kulkarni, S.S.; Newman, A.H. Discovery of heterobicyclic templates for novel metabotropic glutamate receptor subtype 5 antagonists. Bioorg. Med. Chem. Lett 2007, 17, 2987–2991. [Google Scholar]
- Komoriya, S.; Kobayashi, S.; Osanai, K.; Yoshino, T.; Nagata, T.; Haginoya, N.; Nakamoto, Y.; Mochizuki, A.; Nagahara, T.; Suzuki, M.; et al. Design, synthesis, and biological activity of novel factor Xa inhibitors: Improving metabolic stability by S1 and S4 ligand modification. Bioorg. Med. Chem 2006, 14, 1309–1330. [Google Scholar]
- Walczynski, K.; Zuiderveld, O.P.; Timmerman, H. Non-imidazole histamine H3 ligands. Part III. New 4-n-propylpiperazines as non-imidazole histamine H3-antagonists. Eur. J. Med. Chem 2005, 40, 15–23. [Google Scholar]
- Litvinov, V.P.; Dozenko, V.V.; Krivokolysko, S.G. The Chemistry of Thienopyridines. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Elsevier Academic: Amsterdam, The Netherlands, 2007; Volume 93, pp. 117–178. [Google Scholar]



© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mabkhot, Y.N.; Barakat, A.; Al-Majid, A.M.; Alamary, A.S.; Al-Nahary, T.T. A Novel and Expedient Approach to New Thiazoles, Thiazolo[3,2-a]pyridines, Dihydrothiophenes, and Hydrazones Incorporating Thieno[2,3-b]thiophene Moiety. Int. J. Mol. Sci. 2012, 13, 5035-5047. https://doi.org/10.3390/ijms13045035
Mabkhot YN, Barakat A, Al-Majid AM, Alamary AS, Al-Nahary TT. A Novel and Expedient Approach to New Thiazoles, Thiazolo[3,2-a]pyridines, Dihydrothiophenes, and Hydrazones Incorporating Thieno[2,3-b]thiophene Moiety. International Journal of Molecular Sciences. 2012; 13(4):5035-5047. https://doi.org/10.3390/ijms13045035
Chicago/Turabian StyleMabkhot, Yahia Nasser, Assem Barakat, Abdullah Mohammad Al-Majid, Abdullah Saleh Alamary, and Taleb T. Al-Nahary. 2012. "A Novel and Expedient Approach to New Thiazoles, Thiazolo[3,2-a]pyridines, Dihydrothiophenes, and Hydrazones Incorporating Thieno[2,3-b]thiophene Moiety" International Journal of Molecular Sciences 13, no. 4: 5035-5047. https://doi.org/10.3390/ijms13045035
APA StyleMabkhot, Y. N., Barakat, A., Al-Majid, A. M., Alamary, A. S., & Al-Nahary, T. T. (2012). A Novel and Expedient Approach to New Thiazoles, Thiazolo[3,2-a]pyridines, Dihydrothiophenes, and Hydrazones Incorporating Thieno[2,3-b]thiophene Moiety. International Journal of Molecular Sciences, 13(4), 5035-5047. https://doi.org/10.3390/ijms13045035