Optimization of Ligninolytic Enzyme Activity and Production Rate with Ceriporiopsis subvermispora for Application in Bioremediation by Varying Submerged Media Composition and Growth Immobilization Support
Abstract
:1. Introduction
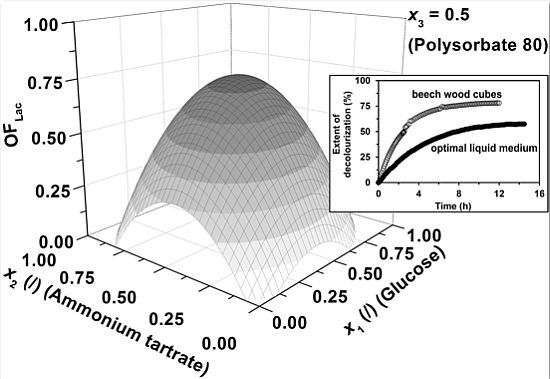
2. Results and Discussion
3. Experimental Section
3.1. Microorganism
3.2. Batch Experiments
3.3. Enzyme Activity
3.4. Response Surface Methodology
3.5. Experiments on Immobilization Supports
3.6. Dyes and Dye Decolourization
4. Conclusions
Acknowledgments
References
- Martínez, A.T.; Speranza, M.; Ruiz-Dueñas, F.J.; Ferreira, P.; Camarero, S.; Guillén, F.; Martínez, M.J.; Gutiérrez, A.; del Río, J.C. Biodegradation of lignocellulosics: Microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol 2005, 8, 195–204. [Google Scholar]
- Enoki, M.; Watanabe, T.; Nakagame, S.; Koller, K.; Messner, K.; Honda, Y.; Kuwahara, M. Extracellular lipid peroxidation of selective white-rot fungus, Ceriporiopsis subvermispora. FEMS Microbiol. Lett 1999, 180, 205–211. [Google Scholar]
- Tanaka, H.; Koike, K.; Itakura, S.; Enoki, A. Degradation of wood and enzyme production by Ceriporiopsis subvermispora. Enzym. Microb. Technol 2009, 45, 384–390. [Google Scholar]
- Maciel, M.J.; Silva, A.C.; Ribeiro, H.C.T. Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: A review. Electron. J. Biotechnol 2010, 13. [Google Scholar] [CrossRef]
- Kirk, T.K.; Cullen, D. Environmentally Friendly Technologies for the Pulp and Paper Industry; Young, R.A., Akhtar, M., Eds.; Wiley: New York, NY, USA, 1998; pp. 273–308. [Google Scholar]
- Blanchette, R.A.; Krueger, E.W.; Haight, J.E.; Akhtar, M.; Akin, D.E. Cell wall alterations in loblolly pinewood decayed by the white-rot fungus Ceriporiopsis subvermispora. J. Biotechnol 1997, 53, 203–213. [Google Scholar]
- Ferraz, A.; Córdova, A.M.; Machuca, A. Wood biodegradation and enzyme production by Ceriporiopsis subvermispora during solid-state fermentation of Eucalyptus grandis. Enzym. Microb. Technol 2003, 32, 59–65. [Google Scholar]
- Trupkin, S.; Levin, L.; Forchiassin, F.; Viale, A. Optimization of a culture medium for ligninolytic enzyme production and synthetic dye decolorization using response surface methodology. J. Ind. Microb. Biotechnol 2003, 30, 682–690. [Google Scholar]
- Levin, L.; Forchiassin, F.; Viale, A. Ligninolytic enzyme production and dye decolorization by Trametes trogii: Application of the Plackett-Burman experimental design to evaluate nutritional requirements. Process Biochem 2005, 40, 1381–1387. [Google Scholar]
- Rigas, F.; Dritsa, V.; Marchant, R.; Papadopoulou, K.; Avramides, E.J.; Hatzianestis, I. Biodegradation of lindane by Pleurotus ostreatus via central composite design. Environ. Int 2005, 31, 191–196. [Google Scholar]
- Liu, L.; Lin, Z.; Zheng, T.; Lin, L.; Zheng, C.; Lin, Z.; Wang, S.; Wang, Z. Fermentation optimization and characterization of the laccase from Pleurotus ostreatus strain 10969. Enzym. Microb. Technol 2009, 44, 426–433. [Google Scholar]
- Tavares, A.P.M.; Coelho, M.A.Z.; Agapito, M.S.M.; Coutinho, J.A.P.; Xavier, A.M.R.B. Optimization and modeling of laccase production by Trametes versicolor in a bioreactor using statistical experimental design. Appl. Biochem. Biotechnol 2006, 134, 233–248. [Google Scholar]
- Quaratino, D.; Ciaffi, M.; Federici, E.; D’annibale, A. Response surface methodology study of laccase production in Panus tigrinus liquid cultures. Biochem. Eng. J 2008, 39, 236–245. [Google Scholar]
- Seker, U.Ö.S.; Catal, T.; Taptık, Y.; Tamerler, C.; Bermek, H. Enhanced production of manganese-peroxidase by the white-rot fungus Bjerkandera adusta using media engineering. Biotechnol. Biotechnol. Eq. 2008, 22, 844–848. [Google Scholar]
- Tavčar, M.; Svobodová, K.; Kuplenk, J.; Novotný, Č.; Pavko, A. Biodegradation of azo dye RO16 in different reactors by immobilized Irpex lacteus. Acta Chim. Slov. 2006, 53, 338–343. [Google Scholar]
- Pavko, A. Fungal Decolourization and Degradation of Synthetic Dyes. Some Chemical Engineering Aspects. In Waste Water—Treatment and Reutilization, EINSCHLAG; García, F.S., Ed.; Rijeka: Intech, Croatia, 2011; pp. 65–88. [Google Scholar]
- Novotný, Č.; Trošt, N.; Šušla, M.; Svobodová, K.; Mikesková, H.; Valková, H.; Malachová, K.; Pavko, A. The use of the fungus Dichomitus squalens for degradation in rotating biological contactor conditions. Bioresour. Technol. 2012, 114, 241–246. [Google Scholar]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: New York, NY, USA, 1989; pp. 384–393. [Google Scholar]
- Souza, T.M.D.; Merritt, C.S.; Reddy, C.A. Lignin-modifying enzymes of the white rot basidiomycete Ganoderma lucidum. Appl. Environ. Microbiol 1999, 65, 5397–5313. [Google Scholar]
- Šušla, M.; Novotný, Č.; Svobodová, K. The implication of Dichomitus squalens laccase isoenzymes in dye decolorization by immobilized fungal cultures. Bioresour. Technol. 2007, 98, 2109–2115. [Google Scholar]
- Babič, J.; Pavko, A. Production of ligninolytic enzymes by Ceriporiopsis subvermispora for decolourization of synthetic dyes. Acta Chim. Slov 2007, 54, 730–734. [Google Scholar]
- Mendonca, R.T.; Jara, J.F.; Gonzales, V.; Ellissetche, J.P.; Freer, F. Evaluation of the white-rot fungi Ganoderma australe and Ceriporiopsis subvermispora in biotechnological applications. J. Ind. Microbiol. Biot 2008, 35, 1323–1330. [Google Scholar]
- Ruttimann-Johnson, C.; Salas, L.; Vicuna, R.; Kirk, T.K. Extracellular enzyme production and synthetic lignin mineralization by Ceriporiopsis subvermispora. Appl. Environ. Microbiol 1993, 59, 1792–1797. [Google Scholar]
- Mikiashvili, N.; Elisashvili, V.; Wasser, S.; Nevo, E. Carbon and nitrogen sources influence the ligninolytic enzyme activity of Trametes versicolor. Biotechnol. Lett 2005, 27, 955–959. [Google Scholar]
- Mikiashvili, N.; Wasser, S.P.; Nevo, E.; Elisashvili, V. Effects of carbon and nitrogen sources on Pleurotus ostreatus ligninolytic enzyme activity. World J. Microbiol. Biotechnol 2006, 22, 999–1002. [Google Scholar]
- Zhang, B.B.; Cheung, P.C. A mechanistic study of the enhancing effect of Tween 80 on the mycelial growth and exopolysaccharide production by Pleurotus tuber-regium. Bioresour. Technol 2011, 102, 8323–8326. [Google Scholar]
- Rayner, A.D.M.; Boddy, L. Fungal Decomposition of Wood. Its Biology and Ecology; John Wiley & Sons: New York, NY, USA, 1988; p. 271. [Google Scholar]
- Liu, W.; Chao, Y.; Yang, X.; Bao, H.; Qian, S. Biodecolorization of azo, anthraquinonic and triphenylmethane dyes by white-rot fungi and a laccase-secreting engineered strain. J. Ind. Microbiol. Biotechnol 2004, 31, 127–132. [Google Scholar]
- Eichlerová, I.; Homolka, L.; Nerud, F. Synthetic dye decolorization capacity of white rot fungus Dichomitus squalens. Bioresour. Technol 2006, 97, 2153–2159. [Google Scholar]
- Tien, M.; Kirk, T.K. Lignin peroxidase from Phanerochaete chrysosporium. Methods Enzymol 1988, 161B, 238–248. [Google Scholar]
- Johannes, C.; Majcherczyk, A.; Hüttermann, A. Degradation of anthracene by laccase of Trametes versicolor in the presence of different mediator compounds. Appl. Microbiol. Biotechnol 1996, 46, 313–317. [Google Scholar]
- Field, J.A.; Vledder, R.H.; van Zelst, J.G.; Rulkens, W.H. The tolerance of lignin peroxidase and manganese-dependent peroxidase to miscible solvents and the in vitro oxidation of anthracene in solvent: Water mixtures. Enzyme Microb. Technol 1996, 18, 300–308. [Google Scholar]










| Absolute variable (setting) value | Normalized variable (setting) value | Maximal Lac activities and production rates | |||||
|---|---|---|---|---|---|---|---|
| Glucose (g L−1) | Ammonium tartrate (g L−1) | Polysorbate 80 (g L−1) | x1 (/) | x2 (/) | x3 (/) | [ELac] (U L−1) | d[ELac]/dt (U L−1 day−1) |
| 2.824 | 0.485 | 0.282 | 0.282 | 0.243 | 0.282 | 32.02 | 10.69 |
| 2.824 | 0.485 | 0.818 | 0.282 | 0.243 | 0.818 | 0.88 | 1.00 |
| 2.824 | 1.615 | 0.282 | 0.282 | 0.807 | 0.282 | 20.67 | 6.64 |
| 2.824 | 1.615 | 0.818 | 0.282 | 0.807 | 0.818 | 3.30 | 1.65 |
| 8.176 | 0.485 | 0.282 | 0.818 | 0.243 | 0.282 | 25.99 | 4.78 |
| 8.176 | 0.485 | 0.818 | 0.818 | 0.243 | 0.818 | 2.40 | 1.20 |
| 8.176 | 1.615 | 0.282 | 0.818 | 0.807 | 0.282 | 40.85 | 8.85 |
| 8.176 | 1.615 | 0.818 | 0.818 | 0.807 | 0.818 | 3.24 | 1.62 |
| 5.500 | 1.050 | 0.550 | 0.550 | 0.525 | 0.550 | 21.36 | 7.64 |
| 5.500 | 1.050 | 0.550 | 0.550 | 0.525 | 0.550 | / | / |
| 5.500 | 1.050 | 0.550 | 0.550 | 0.525 | 0.550 | 29.01 | 5.34 |
| 5.500 | 1.050 | 0.550 | 0.550 | 0.525 | 0.550 | 36.22 | 7.30 |
| 5.500 | 1.050 | 0.550 | 0.550 | 0.525 | 0.550 | / | / |
| 1.000 | 1.050 | 0.550 | 0.100 | 0.525 | 0.550 | 21.05 | 8.70 |
| 10.000 | 1.050 | 0.550 | 1.000 | 0.525 | 0.550 | 3.15 | 1.57 |
| 5.500 | 0.100 | 0.550 | 0.550 | 0.050 | 0.550 | 1.83 | 0.65 |
| 5.500 | 2.000 | 0.550 | 0.550 | 1.000 | 0.550 | 10.48 | 4.92 |
| 5.500 | 1.050 | 0.100 | 0.550 | 0.525 | 0.100 | 41.02 | 15.61 |
| 5.500 | 1.050 | 1.000 | 0.550 | 0.525 | 1.000 | 2.25 | 1.12 |
| 5.500 | 1.050 | 0.550 | 0.550 | 0.525 | 0.550 | 37.03 | 7.66 |
| 5.500 | 1.050 | 0.550 | 0.550 | 0.525 | 0.550 | 24.58 | 6.07 |
| 5.500 | 1.050 | 0.550 | 0.550 | 0.525 | 0.550 | 37.60 | 6.45 |
| 5.500 | 1.050 | 0.550 | 0.550 | 0.525 | 0.550 | 24.12 | 4.19 |
| 5.500 | 1.050 | 0.550 | 0.550 | 0.525 | 0.550 | 30.38 | 4.42 |
| Parameter | Value (i = 1) | Value (i = 2) | Value (i = 3) | Value (i = 4) |
|---|---|---|---|---|
| ai0 | −0.0999 | +1.0141 | +0.1965 | +1.1103 |
| ai1 | +1.9894 | −0.3174 | +1.2765 | +0.1279 |
| ai2 | +2.2481 | +0.8595 | −0.3477 | −0.7177 |
| ai3 | +0.1828 | −1.6619 | −0.9645 | −2.2866 |
| ai12 | +0.9928 | +0.8368 | −1.1417 | −0.3302 |
| ai13 | −0.5400 | +0.4326 | −1.7656 | −1.3103 |
| ai23 | −0.0099 | +0.1115 | +1.0478 | +1.2429 |
| ai11 | −2.1004 | −0.5746 | +0.4619 | +0.7923 |
| ai22 | −2.5276 | −1.1845 | +0.2354 | +0.0892 |
| ai33 | −0.9523 | +0.4468 | +0.7538 | +1.3955 |
| Factor | Normalized | Absolute |
|---|---|---|
| Glucose (g L−1) | 0.198 | 1.980 g L−1 |
| Ammonium tartrate (g L−1) | 0.445 | 0.889 g L−1 |
| Polysorbate 80 (g L−1) | 0.000 | 0.000 g L−1 |
| Factor | Normalized | Absolute |
|---|---|---|
| Glucose (g L−1) | 0.592 | 5.921 g L−1 |
| Ammonium tartrate (g L−1) | 0.562 | 1.126 g L−1 |
| Polysorbate 80 (g L−1) | 0.000 | 0.000 g L−1 |
| Factor | Normalized | Absolute |
|---|---|---|
| Glucose (g L−1) | 0.637 | 6.371 g L−1 |
| Ammonium tartrate (g L−1) | 0.552 | 1.104 g L−1 |
| Polysorbate 80 (g L−1) | 0.000 | 0.000 g L−1 |
| CP (g L−1) | Measured values | Predicted values | ||
|---|---|---|---|---|
| ([ELac])max (U L−1) | ([EMnP])max (U L−1) | ([ELac])max (U L−1) | ([EMnP])max (U L−1) | |
| 0.00 | 5.8 | 89.6 | 28.5 | 85.1 |
| 0.01 | 9.2 | 127.3 | 28.4 | 83.7 |
| 0.05 | 9.5 | 192.8 | 27.7 | 78.1 |
| 0.20 | 4.8 | 146.4 | 26.7 | 71.3 |
| 0.30 | 4.5 | 182.1 | 20.7 | 46.4 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Babič, J.; Likozar, B.; Pavko, A. Optimization of Ligninolytic Enzyme Activity and Production Rate with Ceriporiopsis subvermispora for Application in Bioremediation by Varying Submerged Media Composition and Growth Immobilization Support. Int. J. Mol. Sci. 2012, 13, 11365-11384. https://doi.org/10.3390/ijms130911365
Babič J, Likozar B, Pavko A. Optimization of Ligninolytic Enzyme Activity and Production Rate with Ceriporiopsis subvermispora for Application in Bioremediation by Varying Submerged Media Composition and Growth Immobilization Support. International Journal of Molecular Sciences. 2012; 13(9):11365-11384. https://doi.org/10.3390/ijms130911365
Chicago/Turabian StyleBabič, Janja, Blaž Likozar, and Aleksander Pavko. 2012. "Optimization of Ligninolytic Enzyme Activity and Production Rate with Ceriporiopsis subvermispora for Application in Bioremediation by Varying Submerged Media Composition and Growth Immobilization Support" International Journal of Molecular Sciences 13, no. 9: 11365-11384. https://doi.org/10.3390/ijms130911365
APA StyleBabič, J., Likozar, B., & Pavko, A. (2012). Optimization of Ligninolytic Enzyme Activity and Production Rate with Ceriporiopsis subvermispora for Application in Bioremediation by Varying Submerged Media Composition and Growth Immobilization Support. International Journal of Molecular Sciences, 13(9), 11365-11384. https://doi.org/10.3390/ijms130911365