Experimental Aspects of Colloidal Interactions in Mixed Systems of Liposome and Inorganic Nanoparticle and Their Applications
Abstract
:1. Introduction
1.1. Properties of Phospholipid Bilayers
1.2. Methods of Vesicle Formation
2. Experimental Investigations on Mixed Systems of Liposome and Nanoparticle
2.1. Role of the Lipid Bilayer Phase Behaviour
2.2. Nature of the Interaction in Mixed System Liposome/Nanoparticle
2.2.1. Van der Waals Interaction
2.2.2. Electrostatic Interaction
2.2.3. Hydration Forces
2.2.4. Hydrophobic Interaction
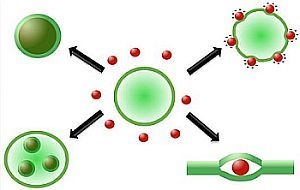
2.3. Structures Encountered in Liposome/Nanoparticle Systems
2.3.1. Supported Lipid Bilayer (SLB) Formation & Particle Internalization
- vesicle-surface interaction;
- vesicle-vesicle interaction, as the lateral interaction between neighbouring vesicles adsorbed on a surface plays an important role in the process of vesicle fusion leading to SLB formation;
- cohesive strength of the vesicle (vesicle stability).
- In the first case, the adsorption of liposomes onto nanoparticles leads to the formation of continuous SLB on the particle surface (Figure 2). This is the case when either the radius of the particle (RNP) is larger than the vesicle radius (RVes) or when the surface area of all particles is equal or larger than the total surface of the potentially formed SLB (SANP ≥ SAVes—taking into account that when SANP ≫ SAVes nanoparticles are believed to be partially covered by lipid bilayer patches [91]).
- In the second case, the formation of SLB on a particle is an intermediate step of the internalization mechanism in which particles covered with SLB become encapsulated within liposomes [42]. This may occur when RVes is considerably larger than RNP and SANP inferior to SAVes (Figure 2). The internalization of the nanoparticles is firstly driven by the attractive interaction between membrane and nanoparticle but in addition, a thermodynamic driving force is the release of water from the vesicle interior and the concomitant entropy gain that occurs during the process.
2.3.2. Decoration and Aggregate Formation
2.3.3. Internalization within the Membrane
3. Applications
3.1. Oxide Nanoparticles (Silica)
3.2. Metallic Nanoparticles (Gold and Silver)
4. Conclusions
Acknowledgments
References
- El-Sayed, M.A. Small is different: Shape-, size-, and composition-dependent properties of some colloidal semiconductor nanocrystals. Acc. Chem. Res 2004, 37, 326–333. [Google Scholar]
- Rao, C.N.R.; Kulkarni, G.U.; Thomas, P.J.; Edwards, P.P. Size-dependent chemistry: Properties of nanocrystals. Chemistry (Weinheim an der Bergstrasse, Germany) 2002, 8, 28–35. [Google Scholar]
- Seo, W.S.; Jo, H.H.; Lee, K.; Kim, B.; Oh, S.J.; Park, J.T. Size-dependent magnetic properties of colloidal Mn3O4 and MnO nanoparticles. Angew. Chem. Int. Ed 2004, 43, 1115–1117. [Google Scholar]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar]
- McFarland, A.D.; van Duyne, R.P. Single silver nanoparticles as real-time optical sensors with zeptomole sensitivity. Nano Lett 2003, 3, 1057–1062. [Google Scholar]
- Shipway, A.N.; Katz, E.; Willner, I. Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. ChemPhysChem 2000, 1, 18–52. [Google Scholar]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med 2007, 3, 95–101. [Google Scholar]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater 2008, 7, 236–241. [Google Scholar]
- Santra, S.; Wang, K.M.; Tapec, R.; Tan, W.H. Development of novel dye-doped silica nanoparticles for biomarker application. J. Biomed. Opt 2001, 6, 160–166. [Google Scholar]
- Tan, W.H.; Wang, K.M.; He, X.X.; Zhao, X.J.; Drake, T.; Wang, L.; Bagwe, R.P. Bionanotechnology based on silica nanoparticles. Med. Res. Rev 2004, 24, 621–638. [Google Scholar]
- Trewyn, B.G.; Slowing, II; Giri, S.; Chen, H.T.; Lin, V.S.Y. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar]
- Bharti, B.; Meissner, J.; Findenegg, G.H. Aggregation of Silica Nanoparticles Directed by Adsorption of Lysozyme. Langmuir 2011, 27, 9823–9833. [Google Scholar]
- Fujita, M.; Katafuchi, Y.; Ito, K.; Kanayama, N.; Takarada, T.; Maeda, M. Structural study on gold nanoparticle functionalized with DNA and its non-cross-linking aggregation. J. Colloid Interface Sci 2012, 368, 629–635. [Google Scholar]
- Lin, Y.F.; Chen, J.H.; Hsu, S.H.; Hsiao, H.C.; Chung, T.W.; Tung, K.L. The synthesis of Lewis acid ZrO2 nanoparticles and their applications in phospholipid adsorption from Jatropha oil used for biofuel. J. Colloid Interface Sci 2012, 368, 660–662. [Google Scholar]
- Fischer, H.C.; Chan, W.C.W. Nanotoxicity: The growing need for in vivo study. Curr. Opin. Biotechnol 2007, 18, 565–571. [Google Scholar]
- Kagan, V.E.; Bayir, H.; Shvedova, A.A. Nanomedicine and nanotoxicology: Two sides of the same coin. Nanomed. Nanotechnol. Biol. Med 2005, 1, 313–316. [Google Scholar]
- Li, Y.F.; Chen, C.Y. Fate and toxicity of metallic and metal-containing nanoparticles for biomedical applications. Small 2011, 7, 2965–2980. [Google Scholar]
- Oberdorster, G.; Stone, V.; Donaldson, K. Toxicology of nanoparticles: A historical perspective. Nanotoxicology 2007, 1, 2–25. [Google Scholar]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of Self-Assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys 1976, 72, 1525–1568. [Google Scholar]
- Kunz, W.; Testard, F.; Zemb, T. Correspondence between curvature, packing parameter, and hydrophilic-lipophilic deviation scales around the phase-inversion temperature. Langmuir 2009, 25, 112–115. [Google Scholar]
- Segota, S.; Tezak, D. Spontaneous formation of vesicles. Adv. Colloid Interface Sci 2006, 121, 51–75. [Google Scholar]
- Marsh, D. General features of phospholipid phase-transitions. Chem. Phys. Lipids 1991, 57, 109–120. [Google Scholar]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol 1965, 13, 238–252. [Google Scholar]
- Gradzielski, M. Vesicles and vesicle gels-structure and dynamics of formation. J. Phys. Condes. Matter 2003, 15, R655–R697. [Google Scholar]
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol 1998, 16, 307–321. [Google Scholar]
- Lasic, D.D.; Papahadjopoulos, D. Medical Applications of Liposomes-Foreword; Elsevier Science, Bv: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev 2004, 56, 675–711. [Google Scholar]
- Jesorka, A.; Orwar, O. Liposomes: Technologies and Analytical Applications. In Annual Review of Analytical Chemistry; Annual Reviews: Palo Alto, CA, USA, 2008; Volume 1, pp. 801–832. [Google Scholar]
- Mabrey, S.; Sturtevant, J.M. Investigation of phase-transitions of lipids and lipid mixtures by high sensitivity differential scanning calorimetry. Proc. Natl. Acad. Sci. USA 1976, 73, 3862–3866. [Google Scholar]
- Janiak, M.J.; Small, D.M.; Shipley, G.G. Nature of thermal pre-transition of synthetic phospholipids - dimyristoyllecithin and dipalmitoyllecithin. Biochemistry 1976, 15, 4575–4580. [Google Scholar]
- Ickenstein, L.M.; Arfvidsson, M.C.; Needham, D.; Mayer, L.D.; Edwards, K. Disc formation in cholesterol-free lippsornes during phase transition. Biochim. Biophys. Acta Biomembr 2003, 1614, 135–138. [Google Scholar]
- Kornberg, R.D.; McConnel, H.M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry 1971, 10, 1111–1120. [Google Scholar]
- Bolinger, P.Y.; Stamou, D.; Vogel, H. Integrated nanoreactor systems: Triggering the release and mixing of compounds inside single vesicles. J. Am. Chem. Soc 2004, 126, 8594–8595. [Google Scholar]
- Watwe, R.M.; Bellare, J.R. Manufacture of liposomes—A review. Curr. Sci 1995, 68, 715–724. [Google Scholar]
- Laughlin, R.G. Equilibrium vesicles: Fact or fiction? Colloid Surf. A Physicochem. Eng. Asp 1997, 128, 27–38. [Google Scholar]
- Gradzielski, M.; Bergmeier, M.; Hoffmann, H.; Muller, M.; Grillo, I. Vesicle gel formed by a self-organization process. J. Phys. Chem. B 2000, 104, 11594–11597. [Google Scholar]
- Jung, H.T.; Lee, S.Y.; Kaler, E.W.; Coldren, B.; Zasadzinski, J.A. Gaussian curvature and the equilibrium among bilayer cylinders, spheres, and discs. Proc. Natl. Acad. Sci. USA 2002, 99, 15318–15322. [Google Scholar]
- Ninham, B.W.; Evans, D.F.; Wei, G.J. The curious world of hydroxide surfactants-spontaneous vesicles and anomalous micelles. J. Phys. Chem 1983, 87, 5020–5025. [Google Scholar]
- Schmolzer, S.; Grabner, D.; Gradzielski, M.; Narayanan, T. Millisecond-range time-resolved small-angle x-ray scattering studies of micellar transformations. Phys. Rev. Lett. 2002, 88. [Google Scholar] [CrossRef]
- Michel, R.; Plostica, T.; Danino, D.; Almasy, L.; Gradzielski, M. Detailed Structure and Electrostatic Properties of Decorated Liposome; Unpublished work; 2012. [Google Scholar]
- Zhang, L.F.; Granick, S. How to stabilize phospholipid liposomes (using nanoparticles). Nano Lett 2006, 6, 694–698. [Google Scholar]
- Lambert, O.; Le Bihan, O.; Bonnafous, P.; Marak, L.; Bickel, T.; Trepout, S.; Mornet, S.; de Haas, F.; Talbot, H.; Taveau, J.C. Cryo-electron tomography of nanoparticle transmigration into liposome. J. Struct. Biol 2009, 168, 419–425. [Google Scholar]
- Savarala, S.; Ahmed, S.; Ilies, M.A.; Wunder, S.L. Stabilization of soft lipid colloids: Competing effects of nanoparticle decoration and supported lipid bilayer formation. ACS Nano 2011, 5, 2619–2628. [Google Scholar]
- Luzzati, V.; Husson, F. Structure of liquid-crystalline phases of lipid-water systems. J. Cell Biol 1962, 12, 207–219. [Google Scholar]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta Rev. Biomembr 2000, 1469, 159–195. [Google Scholar]
- Helfrich, W. Steric interaction of fluid membranes in multilayer systems. Z. Naturfors. Sect. A J. Phys. Sci 1978, 33, 305–315. [Google Scholar]
- Helfrich, W. Elastic properties of lipid bilayers-theory and possible experiments. Z.Naturforsch. (C) 1973, 28, 693–703. [Google Scholar]
- Dobereiner, H.G.; Lipowsky, R. Vesicles in contact with nanoparticles and colloids. Europhys. Lett 1998, 43, 219–225. [Google Scholar]
- Daillant, J.; Bellet-Amalric, E.; Braslau, A.; Charitat, T.; Fragneto, G.; Graner, F.; Mora, S.; Rieutord, F.; Stidder, B. Structure and fluctuations of a single floating lipid bilayer. Proc. Natl. Acad. Sci. USA 2005, 102, 11639–11644. [Google Scholar]
- Lemmich, J.; Mortensen, K.; Ipsen, J.H.; Honger, T.; Bauer, R.; Mouritsen, O.G. Small-angle neutron scattering from multilamellar lipid bilayers: Theory, model, and experiment. Phys. Rev. E 1996, 53, 5169–5180. [Google Scholar]
- Yi, Z.; Nagao, M.; Bossev, D.P. Bending elasticity of saturated and monounsaturated phospholipid membranes studied by the neutron spin echo technique. J. Phys. Condens. Matter Inst. Phys. J 2009, 21, 155104. [Google Scholar]
- Anderson, T.H.; Min, Y.J.; Weirich, K.L.; Zeng, H.B.; Fygenson, D.; Israelachvili, J.N. Formation of supported bilayers on silica substrates. Langmuir 2009, 25, 6997–7005. [Google Scholar]
- Briganti, G.; Cametti, C.; Castelli, F.; Raudino, A. Dielectric behavior of lipid vesicles: The case of L-alpha-Dipalmitoylphosphatidylcholine vesicles as a function of size and temperature. Langmuir 2007, 23, 7518–7525. [Google Scholar]
- Schrader, W.; Halstenberg, S.; Behrends, R.; Kaatze, U. Critical slowing in lipid bilayers. J. Phys. Chem. B 2003, 107, 14457–14463. [Google Scholar]
- Israelachvili, J. Intermolecular and Surface Forces, 3rd ed; Academic Press: London, UK; p. 2011.
- Tadmor, R. The London-van der Waals interaction energy between objects of various geometries. J. Phys. Condes. Matter 2001, 13, L195–L202. [Google Scholar]
- Israelachvili, J.N. Calculation of vanderwaals dispersion forces between macroscopic bodies. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci 1972, 331, 39–55. [Google Scholar]
- Cremer, P.S.; Boxer, S.G. Formation and spreading of lipid bilayers on planar glass supports. J. Phys. Chem. B 1999, 103, 2554–2559. [Google Scholar]
- Radler, J.; Strey, H.; Sackmann, E. Phenomenology and kinetics of lipid bilayer spreading on hydrophilic surfaces. Langmuir 1995, 11, 4539–4548. [Google Scholar]
- Hogg, R.; Healy, T.W.; Fuersten, D.W. Mutual coagulation of colloidal dispersions. Trans. Faraday Soc 1966, 62, 1638–1651. [Google Scholar]
- Ohshima, H.; Healy, T.W.; White, L.R. Improvement on the Hogg-Healy-Fuerstenau Formulas for the Interaction of Dissimilar Double-Layers.1. 2nd and 3rd Approximations for Moderate Potentials. J. Colloid Interface Sci 1982, 89, 484–493. [Google Scholar]
- Parsegian, V.A.; Gingell, D. Electrostatic interaction across a salt solution between 2 bodies bearing unequal charges. Biophys. J. 1972, 12, 1192–1204. [Google Scholar]
- Oleson, T.A.; Sahai, N. Interaction energies between oxide surfaces and multiple phosphatidylcholine bilayers from extended-DLVO theory. J. Colloid Interface Sci 2010, 352, 316–326. [Google Scholar]
- Tero, R.; Ujihara, T.; Urisut, T. Lipid bilayer membrane with atomic step structure: Supported bilayer on a step-and-terrace TiO2(100) surface. Langmuir 2008, 24, 11567–11576. [Google Scholar]
- Leikin, S.; Parsegian, V.A.; Rau, D.C.; Rand, R.P. Hydration forces. Annu. Rev. Phys. Chem 1993, 44, 369–395. [Google Scholar]
- Ohki, S.; Ohshima, H. Interaction and aggregation of lipid vesicles (DLVO theory versus modified DLVO theory). Colloids Surf. B 1999, 14, 27–45. [Google Scholar]
- Israelachvili, J.; Wennerstrom, H. Role of hydration and water structure in biological and colloidal interactions. Nature 1996, 379, 219–225. [Google Scholar]
- Ip, S.; MacLaughlin, C.M.; Gunari, N.; Walker, G.C. Phospholipid membrane encapsulation of nanoparticles for surface-enhanced raman scattering. Langmuir 2011, 27, 7024–7033. [Google Scholar]
- Horn, R.G.; Israelachvili, J.N.; Marra, J.; Parsegian, V.A.; Rand, R.P. Comparison of forces measured between phosphatidylcholine bilayers. Biophys. J 1988, 54, 1185–1186. [Google Scholar]
- Parsegian, V.A.; Rand, R.P.; Fuller, N.L. Direct osmotic-stress measurements of hydration and electrostatic double-layer forces between bilayers of double-chained ammonium acetate surfactants. J. Phys. Chem 1991, 95, 4777–4782. [Google Scholar]
- Rasch, M.R.; Rossinyol, E.; Hueso, J.L.; Goodfellow, B.W.; Arbiol, J.; Korgel, B.A. hydrophobic gold nanoparticle self-assembly with phosphatidylcholine lipid: Membrane-loaded and janus vesicles. Nano Lett 2010, 10, 3733–3739. [Google Scholar]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar]
- Bayerl, T.M.; Bloom, M. Physical-properties of single phospholipid-bilayers adsorbed to micro glass-beads—A new vesicular model system studied by h-2-nuclear magnetic-resonance. Biophys. J 1990, 58, 357–362. [Google Scholar]
- Cha, T.; Guo, A.; Zhu, X.Y. Formation of supported phospholipid bilayers on molecular surfaces: Role of surface charge density and electrostatic interaction. Biophys. J 2006, 90, 1270–1274. [Google Scholar]
- Cho, N.J.; Jackman, J.A.; Liu, M.; Frank, C.W. pH-Driven assembly of various supported lipid platforms: A comparative study on silicon oxide and titanium oxide. Langmuir 2011, 27, 3739–3748. [Google Scholar]
- Czolkos, I.; Jesorka, A.; Orwar, O. Molecular phospholipid films on solid supports. Soft Matter 2011, 7, 4562–4576. [Google Scholar]
- Helm, C.A.; Israelachvili, J.N.; McGuiggan, P.M. Molecular mechanisms and forces involved in the adhesion and fusion of amphiphilic bilayers. Science 1989, 246, 919–922. [Google Scholar]
- Reimhult, E.; Hook, F.; Kasemo, B. Vesicle adsorption on SiO2 and TiO2: Dependence on vesicle size. J. Chem. Phys 2002, 117, 7401–7404. [Google Scholar]
- Richter, R.; Mukhopadhyay, A.; Brisson, A. Pathways of lipid vesicle deposition on solid surfaces: A combined QCM-D and AFM study. Biophys. J 2003, 85, 3035–3047. [Google Scholar]
- Seifert, U.; Lipowsky, R. Adhesion of Vesicles. Phys. Rev. A 1990, 42, 4768–4771. [Google Scholar]
- Min, Y.J.; Pesika, N.; Zasadzinski, J.; Israelachvili, J. Studies of bilayers and vesicle adsorption to solid substrates: Development of a miniature Streaming Potential Apparatus (SPA). Langmuir 2010, 26, 8684–8689. [Google Scholar]
- Goksu, E.I.; Vanegas, J.M.; Blanchette, C.D.; Lin, W.C.; Longo, M.L. AFM for structure and dynamics of biomembranes. Biochim. Biophys. Acta Biomembr 2009, 1788, 254–266. [Google Scholar]
- Mornet, S.; Lambert, O.; Duguet, E.; Brisson, A. The formation of supported lipid bilayers on silica nanoparticles revealed by cryoelectron microscopy. Nano Lett 2005, 5, 281–285. [Google Scholar]
- Moura, S.P.; Carmona-Ribeiro, A.M. Biomimetic particles: Optimization of phospholipid bilayer coverage on silica and colloid stabilization. Langmuir 2005, 21, 10160–10164. [Google Scholar]
- Savarala, S.; Ahmed, S.; Ilies, M.A.; Wunder, S.L. Formation and colloidal stability of DMPC supported lipid bilayers on SiO(2) nanobeads. Langmuir 2010, 26, 12081–12088. [Google Scholar]
- Richter, R.P.; Berat, R.; Brisson, A.R. Formation of solid-supported lipid bilayers: An integrated view. Langmuir 2006, 22, 3497–3505. [Google Scholar]
- Richter, R.P.; Brisson, A.R. Following the formation of supported lipid bilayers on mica: A study combining AFM, QCM-D, and ellipsometry. Biophys. J 2005, 88, 3422–3433. [Google Scholar]
- Seantier, B.; Kasemo, B. Influence of mono- and divalent ions on the formation of supported phospholipid bilayers via vesicle adsorption. Langmuir 2009, 25, 5767–5772. [Google Scholar]
- Reviakine, I.; Brisson, A. Formation of supported phospholipid bilayers from unilamellar vesicles investigated by atomic force microscopy. Langmuir 2000, 16, 1806–1815. [Google Scholar]
- Ahmed, S.; Madathingal, R.R.; Wunder, S.L.; Chen, Y.J.; Bothun, G. Hydration repulsion effects on the formation of supported lipid bilayers. Soft Matter 2011, 7, 1936–1947. [Google Scholar]
- Savarala, S.; Monson, F.; Ilies, M.A.; Wunder, S.L. Supported lipid bilayer nanosystems: Stabilization by undulatory-protrusion forces and destabilization by lipid bridging. Langmuir 2011, 27, 5850–5861. [Google Scholar]
- Roiter, Y.; Ornatska, M.; Rammohan, A.R.; Balakrishnan, J.; Heine, D.R.; Minko, S. Interaction of nanoparticles with lipid membrane. Nano Lett 2008, 8, 941–944. [Google Scholar]
- Roiter, Y.; Ornatska, M.; Rammohan, A.R.; Balakrishnan, J.; Heine, D.R.; Minko, S. Interaction of Lipid Membrane with Nanostructured Surfaces. Langmuir 2009, 25, 6287–6299. [Google Scholar]
- Seifert, U. Configurations of fluid membranes and vesicles. Adv. Phys 1997, 46, 13–137. [Google Scholar]
- Deserno, M.; Gelbart, W.M. Adhesion and wrapping in colloid-vesicle complexes. J. Phys. Chem. B 2002, 106, 5543–5552. [Google Scholar]
- Fleck, C.C.; Netz, R.R. Electrostatic colloid-membrane binding. Europhys. Lett 2004, 67, 314–320. [Google Scholar]
- Deserno, M. When do fluid membranes engulf sticky colloids? J. Phys. Condes. Matter 2004, 16, S2061–S2070. [Google Scholar]
- Deserno, M.; Bickel, T. Wrapping of a spherical colloid by a fluid membrane. Europhys. Lett 2003, 62, 767–773. [Google Scholar]
- Gradzielski, M.; Langevin, D.; Sottmann, T.; Strey, R. Droplet microemulsions at the emulsification boundary: The influence of the surfactant structure on the elastic constants of the amphiphillic film. J. Chem. Phys 1997, 106, 8232–8238. [Google Scholar]
- Safinya, C.R.; Sirota, E.B.; Roux, D.; Smith, G.S. Universality in interacting membranes—The effect of cosurfactants on the interfacial rigidity. Phys. Rev. Lett 1989, 62, 1134–1137. [Google Scholar]
- Szleifer, I.; Kramer, D.; Benshaul, A.; Gelbart, W.M.; Safran, S.A. Molecular theory of curvature elasticity in surfactant films. J. Chem. Phys 1990, 92, 6800–6817. [Google Scholar]
- Ahmed, S.; Wunder, S.L. Effect of high surface curvature on the main phase transition of supported phospholipid bilayers on SiO(2) nanoparticles. Langmuir 2009, 25, 3682–3691. [Google Scholar]
- Westerhausen, C.; Strobl, F.G.; Herrmann, R.; Bauer, A.T.; Schneider, S.W.; Reller, A.; Wixforth, A.; Schneider, A.M.F. Chemical and mechanical impact of silica nanoparticles on the phase transition behavior of phospholipid membranes in theory and experiment. Biophys. J 2012, 102, 1032–1038. [Google Scholar]
- Brocca, P.; Cantu, L.; Corti, M.; Del Favero, E.; Motta, S.; Nodari, M.C. Curved single-bilayers in the region of the anomalous swelling: Effect of curvature and chain length. Colloid Surf. A Physicochem. Eng. Asp 2006, 291, 63–68. [Google Scholar]
- Gaber, B.P.; Peticolas, W.L. On the quantitative interpretation of biomembrane structure by raman-spectroscopy. Biochim. Et Biophys. Acta 1977, 465, 260–274. [Google Scholar]
- Ahmed, S.; Nikolov, Z.; Wunder, S.L. Effect of curvature on nanoparticle supported lipid bilayers investigated by raman spectroscopy. J. Phys. Chem. B 2011, 115, 13181–13190. [Google Scholar]
- Slater, J.L.; Huang, C.H. Interdigitated bilayer-membranes. Prog. Lipid. Res 1988, 27, 325–359. [Google Scholar]
- Mukherjee, S.; Ghosh, R.N.; Maxfield, F.R. Endocytosis. Physiol. Rev 1997, 77, 759–803. [Google Scholar]
- Pelkmans, L.; Puntener, D.; Helenius, A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 2002, 296, 535–539. [Google Scholar]
- Vacha, R.; Martinez-Veracoechea, F.J.; Frenkel, D. Receptor-mediated endocytosis of nanoparticles of various shapes. Nano Lett 2011, 11, 5391–5395. [Google Scholar]
- Smith, K.A.; Jasnow, D.; Balazs, A.C. Designing synthetic vesicles that engulf nanoscopic particles. J. Chem. Phys 2007, 127, 084703:1–084703:10. [Google Scholar]
- Wang, B.; Zhang, L.F.; Bae, S.C.; Granick, S. Nanoparticle-induced surface reconstruction of phospholipid membranes. Proc. Natl. Acad. Sci. USA 2008, 105, 18171–18175. [Google Scholar]
- De Planque, M.R.R.; Aghdaei, S.; Roose, T.; Morgan, H. Electrophysiological Characterization of Membrane Disruption by Nanoparticles. ACS Nano 2011, 5, 3599–3606. [Google Scholar]
- Yu, Y.; Anthony, S.M.; Zhang, L.F.; Bae, S.C.; Granick, S. Cationic nanoparticles stabilize zwitterionic liposomes better than anionic ones. J. Phys. Chem. C 2007, 111, 8233–8236. [Google Scholar]
- Michel, R.; Danino, D.; Gradzielski, M. Internalisation of SiO2 nanoparticles within phospholipid vesicles; Unpublished work; 2012. [Google Scholar]
- Tohver, V.; Smay, J.E.; Braem, A.; Braun, P.V.; Lewis, J.A. Nanoparticle halos: A new colloid stabilization mechanism. Proc. Natl. Acad. Sci. USA 2001, 98, 8950–8954. [Google Scholar]
- Liu, J.; Luijten, E. Colloidal stabilization via nanoparticle halo formation. Phys. Rev. E 2005, 72, 061401:1–061401:10. [Google Scholar]
- Liu, J.W.; Luijten, E. Stabilization of colloidal suspensions by means of highly charged nanoparticles. Phys. Rev. Lett. 2004, 93, 247802:1–247802:4. [Google Scholar]
- Bartucci, R.; Pantusa, M.; Marsh, D.; Sportelli, L. Interaction of human serum albumin with membranes containing polymer-grafted lipids: Spin-label ESR studies in the mushroom and brush regimes. Biochim. Biophys. Acta Biomembr 2002, 1564, 237–242. [Google Scholar]
- Ishihara, A.; Yamauchi, M.; Kusano, H.; Mimura, Y.; Nakakura, M.; Kamiya, M.; Katagiri, A.; Kawano, M.; Nemoto, H.; Suzawa, T.; et al. Preparation and properties of branched oligoglycerol modifiers for stabilization of liposomes. Int. J. Pharm 2010, 391, 237–243. [Google Scholar]
- Woodle, M.C.; Lasic, D.D. Sterically stabilized liposomes. Biochim. Et Biophys. Acta 1992, 1113, 171–199. [Google Scholar]
- Zhang, L.F.; Dammann, K.; Bae, S.C.; Granick, S. Ligand-receptor binding on nanoparticle-stabilized liposome surfaces. Soft Matter 2007, 3, 551–553. [Google Scholar]
- Mohanraj, V.J.; Barnes, T.J.; Prestidge, C.A. Silica nanoparticle coated liposomes: A new type of hybrid nanocapsule for proteins. Int. J. Pharm 2010, 392, 285–293. [Google Scholar]
- Zhang, L.F.; Hong, L.; Yu, Y.; Bae, S.C.; Granick, S. Nanoparticle-assisted surface immobilization of phospholipid liposomes. J. Am. Chem. Soc 2006, 128, 9026–9027. [Google Scholar]
- Jang, H.; Pell, L.E.; Korgel, B.A.; English, D.S. Photoluminescence quenching of silicon nanoparticles in phospholipid vesicle bilayers. J. Photochem. Photobiol. A Chem 2003, 158, 111–117. [Google Scholar]
- Park, S.H.; Oh, S.G.; Mun, J.Y.; Han, S.S. Loading of gold nanoparticles inside the DPPC bilayers of liposome and their effects on membrane fluidities. Colloid Surf. B Biointerfaces 2006, 48, 112–118. [Google Scholar]
- Paasonen, L.; Sipila, T.; Subrizi, A.; Laurinmaki, P.; Butcher, S.J.; Rappolt, M.; Yaghmur, A.; Urtti, A.; Yliperttula, M. Gold-embedded photosensitive liposomes for drug delivery: Triggering mechanism and intracellular release. J. Control. Release 2010, 147, 136–143. [Google Scholar]
- Binder, W.H.; Sachsenhofer, R.; Farnik, D.; Blaas, D. Guiding the location of nanoparticles into vesicular structures: A morphological study. Phys. Chem. Chem. Phys 2007, 9, 6435–6441. [Google Scholar]
- Von White, G.; Chen, Y.J.; Roder-Hanna, J.; Bothun, G.D.; Kitchens, C.L. Structural and Thermal Analysis of Lipid Vesicles Encapsulating Hydrophobic Gold Nanoparticles. ACS Nano 2012, 6, 4678–4685. [Google Scholar]
- Bothun, G.D.; Rabideau, A.E.; Stoner, M.A. Hepatoma cell uptake of cationic multifluorescent quantum dot liposomes. J. Phys. Chem. B 2009, 113, 7725–7728. [Google Scholar]
- Park, S.H.; Oh, S.G.; Mun, J.Y.; Han, S.S. Effects of silver nanoparticles on the fluidity of bilayer in phospholipid liposome. Colloid Surf. B Biointerfaces 2005, 44, 117–122. [Google Scholar]
- Krack, M.; Hohenberg, H.; Kornowski, A.; Lindner, P.; Weller, H.; Forster, S. Nanoparticle-loaded magnetophoretic vesicles. J. Am. Chem. Soc 2008, 130, 7315–7320. [Google Scholar]
- Amstad, E.; Kohlbrecher, J.; Muller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett 2011, 11, 1664–1670. [Google Scholar]
- Al-Jamal, W.T.; Al-Jamal, K.T.; Tian, B.; Lacerda, L.; Bornans, P.H.; Frederik, P.M.; Kostarelos, K. Lipid-quanturn dot bilayer vesicles enhance tumor cell uptake and retention in vitro and in vivo. ACS Nano 2008, 2, 408–418. [Google Scholar]
- Gopalakrishnan, G.; Danelon, C.; Izewska, P.; Prummer, M.; Bolinger, P.Y.; Geissbuhler, I.; Demurtas, D.; Dubochet, J.; Vogel, H. Multifunctional lipid/quantum dot hybrid nanocontainers for controlled targeting of live cells. Angew. Chem. Int. Ed 2006, 45, 5478–5483. [Google Scholar]
- Bothun, G.D. Hydrophobic silver nanoparticles trapped in lipid bilayers: Size distribution, bilayer phase behavior, and optical properties. J. Nanobiotechnol 2008, 6, 13. [Google Scholar]
- Chen, Y.J.; Bose, A.; Bothun, G.D. Controlled release from bilayer-decorated magnetoliposomes via electromagnetic heating. ACS Nano 2010, 4, 3215–3221. [Google Scholar]
- Ginzburg, V.V.; Balijepailli, S. Modeling the thermodynamics of the interaction of nanoparticles with cell membranes. Nano Lett 2007, 7, 3716–3722. [Google Scholar]
- Wi, H.S.; Lee, K.; Pak, H.K. Interfacial energy consideration in the organization of a quantum dot-lipid mixed system. J. Phys. Condes. Matter 2008, 20, 494211:1–494211:6. [Google Scholar]
- Chibowski, E.; Delgado, A.V.; Rudzka, K.; Szczes, A.; Holysz, L. Surface modification of glass plates and silica particles by phospholipid adsorption. J. Colloid Interface Sci 2011, 353, 281–289. [Google Scholar]
- Lukacova, V.; Peng, M.; Fanucci, G.; Tandlich, R.; Hinderliter, A.; Maity, B.; Manivannan, E.; Cook, G.R.; Balaz, S. Drug-membrane interactions studied in phospholipid monolayers adsorbed on nonporous alkylated microspheres. J. Biomol. Screen 2007, 12, 186–202. [Google Scholar]
- Park, J.W. Curvature effect on nanometer-scale surface properties of phospholipid layers. Colloid Surf. B Biointerfaces 2011, 86, 166–168. [Google Scholar]
- Fang, R.H.; Aryal, S.; Hu, C.M.J.; Zhang, L.F. Quick synthesis of lipid-polymer hybrid nanoparticles with low polydispersity using a single-step sonication method. Langmuir 2010, 26, 16958–16962. [Google Scholar]
- Wean Sin, C.; Hadinoto, K. Factors affecting drug encapsulation and stability of lipid-polymer hybrid nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 214–220. [Google Scholar]
- Zhang, L.F.; Chan, J.M.; Gu, F.X.; Rhee, J.W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid-polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar]
- Pidgeon, C.; Venkataram, U.V. Immobilized artificial membrane chromatography-supports composed of membrane-lipids. Anal. Biochem 1989, 176, 36–47. [Google Scholar]
- Winger, T.M.; Chaikof, E.L. Synthesis and characterization of supported phospholipid monolayers: A correlative investigation by radiochemical titration and atomic force microscopy. Langmuir 1998, 14, 4148–4155. [Google Scholar]
- Bothun, G.D.; Preiss, M.R. Bilayer heating in magnetite nanoparticle-liposome dispersions via fluorescence anisotropy. J. Colloid Interface Sci 2011, 357, 70–74. [Google Scholar]
- Rivera Gil, P.; Oberdorster, G.; Elder, A.; Puntes, V.; Parak, W.J. Correlating physico-chemical with toxicological properties of nanoparticles: The present and the future. ACS Nano 2010, 4, 5527–5531. [Google Scholar]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol 2006, 40, 4374–4381. [Google Scholar]
- Chen, J.M.; Hessler, J.A.; Putchakayala, K.; Panama, B.K.; Khan, D.P.; Hong, S.; Mullen, D.G.; DiMaggio, S.C.; Som, A.; Tew, G.N.; et al. Cationic nanoparticles induce nanoscale disruption in living cell plasma membranes. J. Phys. Chem. B 2009, 113, 11179–11185. [Google Scholar]
- Hart, G.A.; Hesterberg, T.W. In vitro toxicity of respirable-size particles of diatomaceous earth and crystalline silica compared with asbestos and titanium dioxide. J. Occup. Environ. Med 1998, 40, 29–42. [Google Scholar]
- Yamamoto, A.; Honma, R.; Sumita, M.; Hanawa, T. Cytotoxicity evaluation of ceramic particles of different sizes and shapes. J. Biomed. Mater. Res. Part A 2004, 68A, 244–256. [Google Scholar]
- Meunier, C.F.; Dandoy, P.; Su, B.L. Encapsulation of cells within silica matrixes: Towards a new advance in the conception of living hybrid materials. J. Colloid Interface Sci 2010, 342, 211–224. [Google Scholar]
- Santra, S.; Tapec, R.; Theodoropoulou, N.; Dobson, J.; Hebard, A.; Tan, W.H. Synthesis and characterization of silica-coated iron oxide nanoparticles in microemulsion: The effect of nonionic surfactants. Langmuir 2001, 17, 2900–2906. [Google Scholar]
- Stober, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci 1968, 26, 62–69. [Google Scholar]
- Slowing, II; Trewyn, B.G.; Giri, S.; Lin, V.S.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar]
- Slowing, II; Trewyn, B.G.; Lin, V.S.Y. Mesoporous silica nanoparticles for intracellular delivery of membrane-impermeable proteins. J. Am. Chem. Soc. 2007, 129, 8845–8849. [Google Scholar]
- Slowing, II; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar]
- Trewyn, B.G.; Giri, S.; Slowing, II; Lin, V.S.Y. Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem. Commun 2007, 3236–3245. [Google Scholar]
- Wang, Y.J.; Caruso, F. Enzyme encapsulation in nanoporous silica spherest. Chem. Commun 2004, 13. [Google Scholar] [CrossRef]
- Giri, S.; Trewyn, B.G.; Stellmaker, M.P.; Lin, V.S.Y. Stimuli-responsive controlled-release delivery system based on mesoporous silica nanorods capped with magnetic nanoparticles. Angew. Chem. Int. Edit 2005, 44, 5038–5044. [Google Scholar]
- Lai, C.Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S.Y. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc 2003, 125, 4451–4459. [Google Scholar]
- Liu, J.W.; Jiang, X.M.; Ashley, C.; Brinker, C.J. Electrostatically mediated liposome fusion and lipid exchange with a nanoparticle-supported bilayer for control of surface charge, drug containment, and delivery. J. Am. Chem. Soc 2009, 131, 7567–7569. [Google Scholar]
- Liu, J.W.; Stace-Naughton, A.; Brinker, C.J. Silica nanoparticle supported lipid bilayers for gene delivery. Chem. Commun 2009. [Google Scholar] [CrossRef]
- Begu, S.; Durand, R.; Lerner, D.A.; Charnay, C.; Tourne-Peteilh, C.; Devoisselle, J.M. Preparation and characterization of siliceous material using liposomes as template. Chem. Commun 2003, 5. [Google Scholar] [CrossRef]
- Begu, S.; Girod, S.; Lerner, D.A.; Jardiller, N.; Tourne-Peteilh, C.; Devoisselle, J.M. Characterization of a phospholipid bilayer entrapped into non-porous silica nanospheres. J. Mater. Chem 2004, 14, 1316–1320. [Google Scholar]
- Begu, S.; Pouessel, A.A.; Lerner, D.A.; Tourne-Peteilh, C.; Devoisselle, J.M. Liposil, a promising composite material for drug storage and release. J. Control. Release 2007, 118, 1–6. [Google Scholar]
- Shen, S.K.; Kendall, E.; Oliver, A.; Ngassam, V.; Hu, D.D.; Parikh, A.N. Liposil-supported lipid bilayers as a hybrid platform for drug delivery. Soft Matter 2011, 7, 1001–1005. [Google Scholar]
- Urban, A.S.; Fedoruk, M.; Horton, M.R.; Radler, J.; Stefani, F.D.; Feldmann, J. Controlled nanometric phase transitions of phospholipid membranes by plasmonic heating of single gold nanoparticles. Nano Lett 2009, 9, 2903–2908. [Google Scholar]
- Bendix, P.M.; Nader, S.; Reihani, S.; Oddershede, L.B. Direct Measurements of Heating by Electromagnetically Trapped Gold Nanoparticles on Supported Lipid Bilayers. ACS Nano 2010, 4, 2256–2262. [Google Scholar]
- Volodkin, D.V.; Skirtach, A.G.; Mohwald, H. Near-IR remote release from assemblies of liposomes and nanoparticles. Angew. Chem. Int. Ed 2009, 48, 1807–1809. [Google Scholar]
- Zharov, V.P.; Galitovskaya, E.N.; Johnson, C.; Kelly, T. Synergistic enhancement of selective nanophotothermolysis with gold nanoclusters: Potential for cancer therapy (vol 37, pg 219, 2005). Lasers Surg. Med 2005, 37, 329–329. [Google Scholar]
- Zharov, V.P.; Galitovsky, V.; Viegas, M. Photothermal detection of local thermal effects during selective nanophotothermolysis. Appl. Phys. Lett 2003, 83, 4897–4899. [Google Scholar]
- Pitsillides, C.M.; Joe, E.K.; Wei, X.B.; Anderson, R.R.; Lin, C.P. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys. J 2003, 84, 4023–4032. [Google Scholar]
- Skirtach, A.G.; Javier, A.M.; Kreft, O.; Kohler, K.; Alberola, A.P.; Mohwald, H.; Parak, W.J.; Sukhorukov, G.B. Laser-induced release of encapsulated materials inside living cells. Angew. Chem. Int. Ed 2006, 45, 4612–4617. [Google Scholar]
- Gormley, A.J.; Malugin, A.; Ray, A.; Robinson, R.; Ghandehari, H. Biological evaluation of RGDfK-gold nanorod conjugates for prostate cancer treatment. J. Drug Target 2011, 19, 915–924. [Google Scholar]
- Hendren, C.O.; Mesnard, X.; Droge, J.; Wiesner, M.R. Estimating production data for five engineered nanomaterials as a basis for exposure assessment. Environ. Sci. Technol 2011, 45, 2562–2569. [Google Scholar]
- Ren, X.L.; Tang, F.Q. Enhancement effect of Ag-Au nanoparticles on glucose biosensor sensitivity. Acta Chim. Sin 2002, 60, 393–397. [Google Scholar]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar]
- Wright, J.B.; Lam, K.; Hansen, D.; Burrell, R.E. Efficacy of topical silver against fungal burn wound pathogens. Am. J. Infect. Control 1999, 27, 344–350. [Google Scholar]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol 2005, 3, 6. [Google Scholar]
- Sun, R.W.Y.; Chen, R.; Chung, N.P.Y.; Ho, C.M.; Lin, C.L.S.; Che, C.M. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem. Commun 2005, 40, 5059–5061. [Google Scholar]
- Jiang, H.Q.; Manolache, S.; Wong, A.C.L.; Denes, F.S. Plasma-enhanced deposition of silver nanoparticles onto polymer and metal surfaces for the generation of antimicrobial characteristics. J. Appl. Polym. Sci 2004, 93, 1411–1422. [Google Scholar]
- Ki, H.Y.; Kim, J.H.; Kwon, S.C.; Jeong, S.H. A study on multifunctional wool textiles treated with nano-sized silver. J. Mater. Sci 2007, 42, 8020–8024. [Google Scholar]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. Vitro 2005, 19, 975–983. [Google Scholar]
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater 2009, 8, 543–557. [Google Scholar]
- Barani, H.; Montazer, M.; Toliyat, T.; Samadi, N. Synthesis of Ag-liposome nano composites. J. Liposome Res 2010, 20, 323–329. [Google Scholar]
- Barani, H.; Montazer, M.; Samadi, N.; Toliyat, T. Nano silver entrapped in phospholipids membrane: Synthesis, characteristics and antibacterial kinetics. Mol. Membr. Biol 2011, 28, 206–215. [Google Scholar]
- Zhou, Y.; Kogiso, M.; Asakawa, M.; Dong, S.; Kiyama, R.; Shimizu, T. Antimicrobial nanotubes consisting of ag-embedded peptidic lipid-bilayer membranes as delivery vehicles. Adv. Mater 2009, 21, 1742–1745. [Google Scholar]









| Temperature/°C | 20 | 50 |
|---|---|---|
| VL/Å3 | 1144 | 1232 |
| D/Å | 63.5 | 67 |
| A/Å2 | 47.9 | 64 |
| DB/Å | 47.8 | 38.5 |
| DW/Å | 15.7 | 28.5 |
| DB’/Å | 42.4 | 46.5 |
| DW’/Å | 11.1 | 20.5 |
| Lipid | Tm/°C | T/°C | (T − Tm)/°C | κc/kBT |
|---|---|---|---|---|
| 14:0 PC | 24 | 22 | −2 | 100.0 ± 4.99 |
| 24 | 0 | 20.9 ± 0.61 | ||
| 28 | +4 | 13.9 ± 0.24 | ||
| 35 | +11 | 15.3 ± 0.31 | ||
| 45 | +21 | 13.9 ± 0.44 | ||
| 60 | +36 | 8.2 ± 0.12 | ||
| 16:0 PC | 41 | 30 | −11 | 49.6 ± 2.78 |
| 41 | 0 | 36.1 ± 1.49 | ||
| 60 | +19 | 9.5 ± 0.18 | ||
| 18:0 PC | 54 | 40 | −14 | 79.1 ± 3.23 |
| 60 | +6 | 13.6 ± 0.24 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Michel, R.; Gradzielski, M. Experimental Aspects of Colloidal Interactions in Mixed Systems of Liposome and Inorganic Nanoparticle and Their Applications. Int. J. Mol. Sci. 2012, 13, 11610-11642. https://doi.org/10.3390/ijms130911610
Michel R, Gradzielski M. Experimental Aspects of Colloidal Interactions in Mixed Systems of Liposome and Inorganic Nanoparticle and Their Applications. International Journal of Molecular Sciences. 2012; 13(9):11610-11642. https://doi.org/10.3390/ijms130911610
Chicago/Turabian StyleMichel, Raphael, and Michael Gradzielski. 2012. "Experimental Aspects of Colloidal Interactions in Mixed Systems of Liposome and Inorganic Nanoparticle and Their Applications" International Journal of Molecular Sciences 13, no. 9: 11610-11642. https://doi.org/10.3390/ijms130911610
APA StyleMichel, R., & Gradzielski, M. (2012). Experimental Aspects of Colloidal Interactions in Mixed Systems of Liposome and Inorganic Nanoparticle and Their Applications. International Journal of Molecular Sciences, 13(9), 11610-11642. https://doi.org/10.3390/ijms130911610