p27 Is a Critical Prognostic Biomarker in Non-Alcoholic Steatohepatitis-Related Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Patients Characteristics
2.1.2. Expression Profile of p27 and Phospho-p27 in NASH-Related HCC
2.1.3. Association between p27 and Cell Proliferation in NASH-Related HCC
2.1.4. Correlation of p27, Phospho-p27 (T157), and Phospho-p27 (S10) Expression with Clinicopathological Profiles in HCC
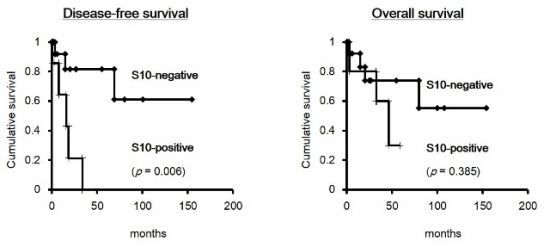
2.1.5. Prognostic Value of the Combination of p27 and Phospho-p27 (S10) in HCC
2.2. Discussion
3. Experimental Section
3.1. Study Subjects
3.2. Immunohistochemistry
3.3. Western Blotting
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. L. Med 2011, 365, 1118–1127. [Google Scholar]
- Matsuda, Y.; Ichida, T.; Fukumoto, M. Hepatocellular carcinoma and liver transplantation: Clinical perspective on molecular targeted strategies. Med. Mol. Morphol 2011, 44, 117–124. [Google Scholar]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar]
- Wakai, T.; Shirai, Y.; Suda, T.; Yokoyama, N.; Sakata, J.; Cruz, P.V.; Kawai, H.; Matsuda, Y.; Watanabe, M.; Aoyagi, Y.; et al. Long-term outcomes of hepatectomy vs. percutaneous ablation for treatment of hepatocellular carcinoma < or =4 cm. World J. Gastroenterol 2006, 12, 546–552. [Google Scholar]
- Wakai, T.; Shirai, Y.; Sakata, J.; Korita, P.V.; Ajioka, Y.; Hatakeyama, K. Surgical outcomes for hepatocellular carcinoma in nonalcoholic fatty liver disease. J. Gastrointest. Surg 2011, 15, 1450–1458. [Google Scholar]
- Lim, K.-C.; Chow, P.K.-H.; Allen, J.C.; Siddiqui, F.J.; Chan, E.S.-Y.; Tan, S.-B. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br. J. Surg 2012, 99, 1622–1629. [Google Scholar]
- Cucchetti, A.; Piscaglia, F.; Cescon, M.; Ercolani, G.; Pinna, A.D. Systematic review of surgical resection vs. radiofrequency ablation for hepatocellular carcinoma. World J. Gastroenterol 2013, 19, 4106–4118. [Google Scholar]
- Zetter, B.R. The cellular basis of site-specific tumor metastasis. N. Engl. L. Med 1990, 322, 605–612. [Google Scholar]
- Qin, L.-X.; Tang, Z.-Y. The prognostic molecular markers in hepatocellular carcinoma. World J. Gastroenterol 2002, 8, 385–392. [Google Scholar]
- Matsuda, Y. Molecular mechanism underlying the functional loss of cyclindependent kinase inhibitors p16 and p27 in hepatocellular carcinoma. World J. Gastroenterol 2008, 14, 1734–1740. [Google Scholar]
- Hui, A.; Sun, L.; Kanai, Y.; Sakamoto, M.; Hirohashi, S. Reduced p27 Kip1 expression in hepatocellular carcinomas. Cancer Lett 1998, 132, 67–73. [Google Scholar]
- Ito, Y.; Matsuura, N.; Sakon, M.; Miyoshi, E.; Noda, K.; Takeda, T.; Umeshita, K.; Nagano, H.; Nakamori, S.; Dono, K.; et al. Expression and prognostic roles of the G1-S modulators in hepatocellular carcinoma: p27 independently predicts the recurrence. Hepatology 1999, 30, 90–99. [Google Scholar]
- Tannapfel, A.; Grund, D.; Katalinic, A.; Uhlmann, D.; Köckerling, F.; Haugwitz, U.; Wasner, M.; Hauss, J.K.; Engeland, C.W. Decreased expression of p27 protein is associated with advanced tumor stage in hepatocellular carcinoma. Int. J. Cancer 2000, 355, 350–355. [Google Scholar]
- Fiorentino, M.; Altimari, A.; Errico, A.D. Acquired expression of p27 is a favorable prognostic indicator in patients with hepatocellular carcinoma acquired expression of p27 is a favorable prognostic indicator in patients with hepatocellular carcinoma. Clin. Cancer Res 2000, 6, 3966–3972. [Google Scholar]
- Nakayama, K.; Ishida, N.; Shirane, M.; Inomata, A.; Inoue, T.; Shishido, N.; Horii, I.; Loh, D.Y. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 1996, 85, 707–720. [Google Scholar]
- Kiyokawa, H.; Kineman, R.D.; Manova-Todorova, K.O.; Soares, V.C.; Hoffman, E.S.; Ono, M.; Khanam, D.; Hayday, A.C.; Frohman, L.A.; Koff, A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 1996, 85, 721–732. [Google Scholar]
- Vervoorts, J.; Lüscher, B. Post-translational regulation of the tumor suppressor p27(KIP1). Cell. Mol. Life Sci 2008, 65, 3255–3264. [Google Scholar]
- Wander, S.A.; Zhao, D.; Slingerland, J.M. P27: A barometer of signaling deregulation and potential predictor of response to targeted therapies. Clin. Cancer Res 2011, 17, 12–18. [Google Scholar]
- Sgambato, A.; Zhang, Y.J.; Arber, N.; Hibshoosh, H.; Doki, Y.; Ciaparrone, M.; Santella, R.M.; Cittadini, A.; Weinstein, I.B. Deregulated expression of p27(Kip1) in human breast cancers. Clin. Cancer Res 1997, 3, 1879–1887. [Google Scholar]
- Fredersdorf, S.; Burns, J.; Milne, A.M.; Packham, G.; Fallis, L.; Gillett, C.E.; Royds, J.A.; Peston, D.; Hall, P.A.; Hanby, A.M.; et al. High level expression of p27(kip1) and cyclin D1 in some human breast cancer cells: Inverse correlation between the expression of p27(kip1) and degree of malignancy in human breast and colorectal cancers. Proc. Natl. Acad. Sci. USA 1997, 94, 6380–6385. [Google Scholar]
- Viglietto, G.; Motti, M.L.; Bruni, P.; Melillo, R.M.; D’Alessio, A.; Califano, D.; Vinci, F.; Chiappetta, G.; Tsichlis, P.; Bellacosa, A.; et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat. Med 2002, 8, 1136–1144. [Google Scholar]
- Matsuda, Y.; Ichida, T.; Genda, T. Loss of p16 Contributes to p27 Sequestration by Cyclin D1-cyclin-dependent kinase 4 complexes and poor prognosis in hepatocellular carcinoma loss of p16 contributes to p27 sequestration by cyclin D1-cyclin-dependent kinase 4 complexes and poor progno. Clin. Cancer Res 2003, 9, 3389–3396. [Google Scholar]
- Nan, K.; Jing, Z.; Gong, L. Expression and altered subcellular localization of the cyclin-dependent kinase inhibitor p27Kip1 in hepatocellular carcinoma. World J. Gastroenterol 2004, 10, 1425–1430. [Google Scholar]
- He, S.; Lu, M.; Xue, W.; Wang, Y.; Zhao, Y.; Gao, S.; Ke, Q.; Liu, Y.; Li, P.; Cui, X.; et al. Phosphorylated p27Kip1 on Thr157 is an important prognosis in human hepatocellular carcinoma in vivo and in vitro. Med. Oncol 2011, 28, 94–104. [Google Scholar]
- Boehm, M.; Yoshimoto, T.; Crook, M.F.; Nallamshetty, S.; True, A.; Nabel, G.J.; Nabel, E.G. A growth factor-dependent nuclear kinase phosphorylates p27(Kip1) and regulates cell cycle progression. EMBO J 2002, 21, 3390–3401. [Google Scholar]
- Rodier, G.; Montagnoli, A.; di Marcotullio, L.; Coulombe, P.; Draetta, G.F.; Pagano, M.; Meloche, S. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J 2001, 20, 6672–6682. [Google Scholar]
- Mcallister, S.S.; Becker-hapak, M.; Pagano, M.; Dowdy, S.F.; Pintucci, G. Novel p27kip1 C-terminal scatter domain mediates rac-dependent cell migration independent of cell cycle arrest functions novel p27 kip1 C-terminal scatter domain mediates rac-dependent cell migration independent of cell cycle arrest functions. Mol. Cell Biol 2003, 23, 216–228. [Google Scholar]
- Yachida, S.; Sakamoto, M.; Imaida, K.; Yokohira, M.; Saoo, K.; Okano, K.; Wakabayashi, H.; Maeta, H.; Suzuki, Y. p27(Kip1) is overexpressed in very early stages of hepatocarcinogenesis. Cancer Sci 2008, 99, 2152–2159. [Google Scholar]
- Lewis, J.R.; Mohanty, S.R. Nonalcoholic fatty liver disease: A review and update. Dig. Dis. Sci 2010, 55, 560–578. [Google Scholar]
- Hashimoto, E.; Tokushige, K. Prevalence, gender, ethnic variations, and prognosis of NASH. J. Gastroenterol 2011, 46, 63–69. [Google Scholar]
- Starley, B.Q.; Calcagno, C.J.; Harrison, S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology 2010, 51, 1820–1832. [Google Scholar]
- Hui, J.M.; Kench, J.G.; Chitturi, S.; Sud, A.; Farrell, G.C.; Byth, K.; Hall, P.; Khan, M.; George, J. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology 2003, 38, 420–427. [Google Scholar]
- Sanyal, A.J.; Banas, C.; Sargeant, C.; Luketic, V.A.; Sterling, R.K.; Stravitz, R.T.; Shiffman, M.L.; Heuman, D.; Coterrell, A.; Fisher, R.A.; et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006, 43, 682–689. [Google Scholar]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.-R.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar]
- Ohki, T.; Tateishi, R.; Shiina, S.; Goto, E.; Sato, T.; Nakagawa, H.; Masuzaki, R.; Goto, T.; Hamamura, K.; Kanai, F.; et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut 2009, 58, 839–844. [Google Scholar]
- Tokushige, K.; Hashimoto, E.; Yatsuji, S.; Tobari, M.; Taniai, M.; Torii, N.; Shiratori, K. Prospective study of hepatocellular carcinoma in nonalcoholic steatohepatitis in comparison with hepatocellular carcinoma caused by chronic hepatitis C. J. Gastroenterol 2010, 45, 960–967. [Google Scholar]
- Reddy, S.K.; Steel, J.L.; Chen, H.-W.; DeMateo, D.J.; Cardinal, J.; Behari, J.; Humar, A.; Marsh, J.W.; Geller, D.A.; Tsung, A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis vs. hepatitis C and alcoholic liver disease. Hepatology 2012, 55, 1809–1819. [Google Scholar]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am. J. Gastroenterol 2012, 107, 811–826. [Google Scholar]
- Cotler, S.J.; Hay, N.; Xie, H.; Chen, M.L.; Xu, P.Z.; Layden, T.J.; Guzman, G. Immunohistochemical expression of components of the Akt-mTORC1 pathway is associated with hepatocellular carcinoma in patients with chronic liver disease. Dig. Dis. Sci 2008, 53, 844–849. [Google Scholar]
- Oliva, J.; French, B.A.; Qing, X.; French, S.W. The identification of stem cells in human liver diseases and hepatocellular carcinoma. Exp. Mol. Pathol 2010, 88, 331–340. [Google Scholar]
- Salomao, M.; Yu, W.M.; Brown, R.S., Jr.; Emond, J.C.; Lefkowitch, J.H. Steatohepatitic hepatocellular carcinoma (SH-HCC): A distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am. J. Surg. Pathol 2010, 34, 1630–1636. [Google Scholar]
- Sharma, D.; Wang, J.; Fu, P.P.; Sharma, S.; Nagalingam, A.; Mells, J.; Handy, J.; Page, A.J.; Cohen, C.; Anania, F.A.; et al. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology 2010, 52, 1713–1722. [Google Scholar]
- Martínez-López, N.; Varela-Rey, M.; Fernández-Ramos, D.; Woodhoo, A.; Vázquez-Chantada, M.; Embade, N.; Espinosa-Hevia, L.; Bustamante, F.J.; Parada, L.A.; Rodriguez, M.S.; et al. Activation of LKB1-Akt pathway independent of phosphoinositide 3-kinase plays a critical role in the proliferation of hepatocellular carcinoma from nonalcoholic steatohepatitis. Hepatology 2010, 52, 1621–1631. [Google Scholar]
- Stefano, J.T.; de Oliveira, C.P.; Corrêa-Giannella, M.L.; Soares, I.C.; Kubrusly, M.S.; Bellodi-Privato, M.; de Mello, E.S.; de Lima, V.M.; Carrilho, F.J.; Alves, V.A. Decreased immunoexpression of survivin could be a potential marker in human non-alcoholic fatty liver disease progression? Liver Int 2011, 31, 377–385. [Google Scholar]
- Tanaka, S.; Miyanishi, K.; Kobune, M.; Kawano, Y.; Hoki, T.; Kubo, T.; Hayashi, T.; Sato, T.; Sato, Y.; Takimoto, R.; et al. Increased hepatic oxidative DNA damage in patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. J. Gastroenterol 2013. [Google Scholar] [CrossRef]
- Kotake, Y.; Nakayama, K.; Ishida, N.; Nakayama, K. Role of serine 10 phosphorylation in p27 stabilization revealed by analysis of p27 knock-in mice harboring a serine 10 mutation. J. Biol. Chem 2005, 280, 1095–1102. [Google Scholar]
- Wang, Y.; Wang, Y.; Cheng, C.; Ji, Y.; Zhao, Y.; Zou, L.; Shen, A. Expression of Jun activation domain-binding protein 1 and Ser10 phosphorylated p27 protein in human epithelial ovarian carcinoma. J. Cancer Res. Clin. Oncol 2009, 135, 951–959. [Google Scholar]
- He, S.-M.; Zhao, Z.-W.; Wang, Y.; Zhao, J.-P.; Wang, L.; Hou, F.; Gao, G.-D. Potential role of Jun activation domain-binding protein 1 and phosphorylated p27 expression in prognosis of glioma. Brain Tumor Pathol 2012, 29, 3–9. [Google Scholar]
- Kleiner, D.E.; Brunt, E.M.; van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar]
- Liver Cancer Study Group of Japan, The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 5th ed.; Kanehara: Tokyo, Japan, 2008; Volume 19.




| Clinical characteristics | Patients | (%) |
|---|---|---|
| Tumor size | ||
| ≤5 | 15 | 68.2 |
| >5 | 7 | 31.8 |
| Vascular invasion | ||
| absent | 17 | 77.3 |
| present | 5 | 22.7 |
| Histological grade | ||
| I | 6 | 27.3 |
| II | 16 | 72.7 |
| Tumor stage | ||
| pT1 | 14 | 63.6 |
| pT2 | 5 | 22.7 |
| pT3 | 3 | 13.6 |
| Tumor recurrence | ||
| absent | 14 | 63.6 |
| present | 8 | 36.4 |
| p27 phosphorylation | p27 expression | ||||
|---|---|---|---|---|---|
| low (n = 13) | high (n = 9) | r | p | ||
| p-p27 (T157) | − | 13 | 5 | ||
| + | 0 | 4 | 0.652 | 0.003 | |
| p-p27 (S10) | − | 8 | 7 | ||
| + | 5 | 2 | 0.193 | 0.378 | |
| Tumor status | p27 | phospho-p27 (T157) | phospho-p27 (S10) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| low | high | p | − | + | p | − | + | p | |
| Tumor size | |||||||||
| ≤5 | 6 | 9 | 11 | 4 | 10 | 4 | |||
| >5 | 7 | 0 | 0.010 | 7 | 0 | 0.187 | 5 | 3 | 0.510 |
| Vascular invasion | |||||||||
| absent | 11 | 6 | 15 | 2 | 11 | 6 | |||
| present | 2 | 3 | 0.316 | 3 | 2 | 0.209 | 4 | 1 | 0.476 |
| Histological grade | |||||||||
| I | 2 | 4 | 3 | 3 | 5 | 1 | |||
| II | 11 | 5 | 0.155 | 15 | 1 | 0.045 | 10 | 6 | 0.349 |
| Tumor stage | |||||||||
| pT1 | 10 | 4 | 13 | 1 | 9 | 5 | |||
| pT2-3 | 3 | 5 | 0.135 | 5 | 3 | 0.116 | 6 | 2 | 0.490 |
| Clinicopathological factors | HR | 95% CI | pa |
|---|---|---|---|
| Tumor size (>5 cm) | 4.109 | 0.795–21.217 | 0.092 |
| Intrahepatic metastasis | 1.021 | 0.197–5.297 | 0.980 |
| Histological grade (II) a | 1.415 | 0.269–7.445 | 0.682 |
| Vascular invasion | 1.768 | 0.341–9.157 | 0.497 |
| Tumor stage (pT2-4) b | 0.673 | 0.129–3.514 | 0.639 |
| p27 (low-expressor) | 6.211 | 0.734–52.581 | 0.094 |
| phospho-p27 (T157) | 0.530 | 0.063–4.449 | 0.559 |
| phospho-p27 (S10) | 7.623 | 1.457–39.882 | 0.016 * |
| Cases a | Age | Gender | DFS b | p27 | p-p27 (T157) | p-p27 (S10) |
|---|---|---|---|---|---|---|
| 1 | 73 | female | 1.7 | low | − | + |
| 2 | 60 | male | 7.8 | low | − | + |
| 3 | 75 | female | 18.5 | low | − | + |
| 4 | 64 | male | 3.7 | low | − | − |
| 5 | 55 | male | 33.6 | low | − | + |
| 6 | 83 | male | 15.9 | high | + | + |
| 7 | 66 | male | 14.9 | low | − | − |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matsuda, Y.; Wakai, T.; Hirose, Y.; Osawa, M.; Fujimaki, S.; Kubota, M. p27 Is a Critical Prognostic Biomarker in Non-Alcoholic Steatohepatitis-Related Hepatocellular Carcinoma. Int. J. Mol. Sci. 2013, 14, 23499-23515. https://doi.org/10.3390/ijms141223499
Matsuda Y, Wakai T, Hirose Y, Osawa M, Fujimaki S, Kubota M. p27 Is a Critical Prognostic Biomarker in Non-Alcoholic Steatohepatitis-Related Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2013; 14(12):23499-23515. https://doi.org/10.3390/ijms141223499
Chicago/Turabian StyleMatsuda, Yasunobu, Toshifumi Wakai, Yuki Hirose, Mami Osawa, Shun Fujimaki, and Masayuki Kubota. 2013. "p27 Is a Critical Prognostic Biomarker in Non-Alcoholic Steatohepatitis-Related Hepatocellular Carcinoma" International Journal of Molecular Sciences 14, no. 12: 23499-23515. https://doi.org/10.3390/ijms141223499
APA StyleMatsuda, Y., Wakai, T., Hirose, Y., Osawa, M., Fujimaki, S., & Kubota, M. (2013). p27 Is a Critical Prognostic Biomarker in Non-Alcoholic Steatohepatitis-Related Hepatocellular Carcinoma. International Journal of Molecular Sciences, 14(12), 23499-23515. https://doi.org/10.3390/ijms141223499