New Mononuclear Cu(II) Complexes and 1D Chains with 4-Amino-4H-1,2,4-triazole
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. IR and Diffuse Reflectance Spectroscopy
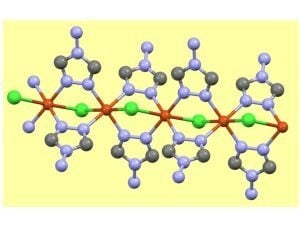
2.3. Structural Aspects
2.4. Magnetic Properties
3. Experimental Section
3.1. General Procedures
3.2. Syntheses
3.3. X-ray Crystallography
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Saini, M.S.; Dwivedi, J. Synthesis and biological significances of 1,2,4-triazole and its derivatives: A review. Int. J. Pharm. Sci. Res 2013, 4, 2866–2879. [Google Scholar]
- Tamura, K.; Inoue, K.; Takahashi, M.; Matsuo, S.; Irie, K.; Kodama, Y.; Ozawa, S.; Nishikawa, A.; Yoshida, M. Dose-response involment of constitutive androstate receptor in mouse liver hypertrophy induced by triazole fungicides. Toxicol. Lett 2013, 221, 47–56. [Google Scholar]
- Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M. Metal azolate frameworks: From crystal engineering to functional materials. Chem. Rev 2012, 112, 1001–1033. [Google Scholar]
- Haasnoot, J.G. Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1,2,4-triazole derivatives as ligands. Coord. Chem. Rev 2000, 200–202, 131–185. [Google Scholar]
- Naik, A.D.; Dîrtu, M.M.; Railliet, A.P.; Marchand-Brynaert, J.; Garcia, Y. Coordination polymers and metal organic frameworks derived from 1,2,4-triazole amino acid linkers. Polymers 2011, 3, 1750–1775. [Google Scholar]
- Wu, X.-Y.; Kuang, X.-F.; Zhao, Z.-G.; Chen, S.-C.; Xie, Y.-M.; Yu, R.-M.; Lu, C.-Z. A series of POM-based hybrid materials with different copper/aminotriazole motif. Inorg. Chim. Acta 2010, 363, 1236–1242. [Google Scholar]
- Zhou, J.-H.; Cheng, R.-M.; Song, Y.; Li, Y.-Z.; Yu, Z.; Chen, X.-T.; Xue, Z.-L.; You, X.-Z. Syntheses, structures, and magnetic properties of unusual nonlinear polynuclear copper(II) complexes containing derivatives of 1,2,4-triazole and pivalate ligands. Inorg. Chem 2005, 44, 8011–8022. [Google Scholar]
- Yang, E.-C.; Ding, B.; Liu, Z.-Y.; Yang, Y.-L.; Zhao, X.-J. Structural transformation from a discrete Cu(II)4 cluster to two extended Cu(II)4 + Cu(II)1 chain-based three-dimensional frameworks by changing the spacer functionality: Synthesis, crystal structures, and magnetic properties. Cryst. Growth Des 2012, 12, 1185–1192. [Google Scholar]
- Yang, E.-C.; Liu, Z.-Y.; Zhang, C.-H.; Yang, Y.-L.; Zhao, X.-J. Structural diversity directed by switchable coordination of substitute groups in a ternary Cu(II)-triazole-sulfoisophthalate self-assembly system: Synthesis, crystal structures and magnetic behavior. Dalton Trans 2013, 42, 1581–1590. [Google Scholar]
- Romanenko, G.V.; Savelieva, Z.A.; Podberezskaya, N.V.; Larionov, S.V. Structure of the Cu(II) chloride complex with 4-amino-l,2,4-triazole CuC2H4N4Cl2. J. Struct. Chem 1997, 38, 171–176. [Google Scholar]
- Liu, J.C.; Fu, D.G.; Zhuang, J.Z.; Duan, C.Y.; You, X.Z. Linear trinuclear and one-dimensional copper(II) complexes containing co-bridging end-on azido and triazole ligands. Crystal structures and magnetic properties of [Cu3(atrz)2(N3)6] and [Cu(atrz)2(N3)]NO3 (atrz = 4-amino-1,2,4-triazole). J. Chem. Soc.-Dalton Trans 1999, 14, 2337–2342. [Google Scholar]
- Ding, B.; Huang, Y.Q.; Liu, Y.Y.; Shi, W.; Cheng, P. Synthesis, structure and magnetic properties of a novel 1D coordination polymer {[Cu2(amtrz)4(1,1-μ-NCS)2](ClO4)2·H2O}n. Inorg. Chem. Commun 2007, 10, 7–10. [Google Scholar]
- Mahenthirarajah, T.; Li, Y.; Lightfoot, P. Organic-inorganic hybrid chains and layers constructed from copper-amine cations and early transition metal (Nb, Mo) oxyfluoride anions. Dalton Trans 2009, 17, 3280–3285. [Google Scholar]
- García-Couceiro, U.; Castillo, O.; Luque, A.; García-Terán, J.P.; Beobide, G.; Román, P. One-dimensional oxalato-bridged metal(II) complexes with 4-amino-1,2,4-triazole as apical ligand. Eur. J. Inorg. Chem 2005. [Google Scholar] [CrossRef]
- Yang, E.-C.; Liu, Z.-Y.; Zhao, L.-N.; Yang, Y.-L.; Zhang, C.-H.; Zhao, X.-J. Ligand-deprotonation induced structural diversity in a ternary Cu(II)-triazole-tetracarboxylate self-assembly system: Synthesis, crystal structures, and magnetic behavior. CrystEngComm 2011, 13, 5401–5408. [Google Scholar]
- Liu, Z.-Y.; Su, Y.-H.; Yang, E.-C.; Zhao, X.-J. Structural transformation from a coplanar layer to a linear chain by switchable coordination of exocyclic amino group. Inorg. Chem. Commun 2012, 26, 56–59. [Google Scholar]
- Qiao, L.-Y.; Zhang, J.; Li, Z.-J.; Qin, Y.-Y.; Yin, P.-X.; Cheng, J.-K.; Yao, Y.-G. Hydrogen-bond directed self-assembly of a novel 2D → 3D polythreading with finite components based on rigid ligand. J. Mol. Struct 2011, 994, 1–5. [Google Scholar]
- Dîrtu, M.M.; Neuhausen, C.; Naik, A.D.; Rotaru, A.; Spinu, L.; Garcia, Y. Insights into the origin of cooperative effects in the spin transition of [Fe(NH2trz)3](NO3)2: The role of supramolecular interactions evidenced in the crystal structure of [Cu(NH2trz)3](NO3)2 H2O. Inorg. Chem 2010, 49, 5723–5736. [Google Scholar]
- Garcia, Y.; Campbell, S.J.; Lord, J.S.; Boland, Y.; Ksenofontov, V.; Gutlich, P. Dynamics and supramolecular organization of the 1D spin transition polymeric chain compound [Fe(NH2trz)3](NO3)2. Muon spin relaxation. J. Phys. Chem. B 2007, 111, 11111–11119. [Google Scholar]
- Grosjean, A.; Daro, N.; Kauffmann, B.; Kaiba, A.; Letard, J.-F.; Guionneau, P. The 1-D polymeric structure of the Fe(NH2trz)3(NO3)2·nH2O (with n = 2) spin crossover compound proven by single crystal investigations. Chem. Commun 2011, 47, 12382–13284. [Google Scholar]
- Drabent, K.; Ciunik, Z. Counter anion dependent symmetry of Cu(II)-4-amino-1,2,4-triazole polymeric chains. Chem. Commun 2001, 14, 1254–1255. [Google Scholar]
- Dîrtu, M.M.; Rotaru, A.; Gillard, D.; Linares, J.; Codjovi, E.; Tinant, B.; Garcia, Y. Prediction of the spin transition temperature in Fe(II) one-dimensional coordination polymers: An anion based database. Inorg. Chem 2009, 48, 7838–7852. [Google Scholar]
- Sinditskii, V.P.; Sokol, V.I.; Fogelzang, A.E.; Dutov, M.D.; Serushkin, V.V.; Poraikoshits, M.A.; Svetlov, B.S. The vibrational Spectra and structure of coordination compounds of metals with 4-amino-1,2,4-triazole as bidendate ligand. Russ. J. Inorg. Chem 32.
- Yi, L.; Du, J.-Y.; Liu, S.; Wang, X.-X. Supramolecular polymers of two novel 4-substituted-1,2,4-triazolate complexes: [Cd(pCltrz)2(NCS)2(H2O)2] and [Cu(4-atrz)4(Cl)0.5(H2O)0.5](ClO4)1.5 (pCltrz: 4-(p-chlorophenyl)-1,2,4-triazole; 4-atrz: 4-amino-1,2,4-triazole). J. Chem. Res 2004, 1, 29–31. [Google Scholar]
- Huheey, J.; Keiter, E.; Keiter, E.A.; Richard, L. Inorganic Chemistry: Principles of Structure and Reactivity; HarperCollins College: New York, NY, USA, 1993. [Google Scholar]
- Jarvis, J. The crystal structure of a complex of cupric chloride and 1:2:4-triazole. Acta Cryst 1962, 15, 964–966. [Google Scholar]
- Garcia, Y.; van Koningsbruggen, P.J.; Bravic, G.; Guionneau, P.; Chasseau, D.; Cascarano, G.L.; Moscovici, J.; Lambert, K.; Michalowicz, A.; Kahn, O. Synthesis, crystal structure, EXAFS, and magnetic properties of catena-poly μ-tris(4-(2-hydroxyethyl)-1,2,4-triazole-N1,N2)copper(II) diperchlorate trihydrate: Relevance with the structure of the iron(II) 1,2,4-triazole spin transition molecular materials. Inorg. Chem 1997, 36, 6357–6365. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst 2008, 41, 466–470. [Google Scholar]
- Garcia, Y.; van Koningsbruggen, P.J.; Bravic, G.; Chasseau, D.; Kahn, O. A Cu(II) chain compound showing a ferromagnetic coupling through triple N1,N2-1,2,4-triazole bridges. Eur. J. Inorg. Chem 2003, 2, 356–362. [Google Scholar]
- Saha, M.K.; Bernal, I. Environment-controlled switching between cyclic hexamer and helical conformations of a water chloride cluster: An old compound viewed in a new perspective. Inorg. Chem. Commun 2005, 8, 871–873. [Google Scholar]
- Bai, S.-Q.; Quek, G.Y.H.; Koh, L.L.; Andy Hor, T. Crystallographic analysis of different water-halide cluster blends in cationic [(SNS)Pd(II)] pincer complexes. CrystEngComm 2010, 12, 226–233. [Google Scholar]
- Emori, S.; Inoue, M.; Kubo, M. Magnetic susceptibilities and broad-line pmr spectra of dichloro(pyridazine)copper(II) and related compounds. Bull. Chem. Soc. Jpn 1972, 45, 2259–2265. [Google Scholar]
- Bonner, J.C.; Fisher, M.E. Linear magnetic chains with anisotropic coupling. Phys. Rev. A 1964, 135, 640–658. [Google Scholar]
- Estes, W.E.; Gavel, D.P.; Hatfield, W.E.; Hodgson, D.J. Magnetic and structural characterization of dibromo- and dichlorobis(thiazole)copper(II). Inorg. Chem 1978, 17, 1415–1421. [Google Scholar]
- Drabent, K.; Ciunik, Z.; Ozarowski, A. X-ray crystal structures, electron paramagnetic resonance, and magnetic studies on strongly antiferromagnetically coupled mixed μ-hydroxide-μ-N1,N2-triazole-bridged one dimensional linear chain copper(II) complexes. Inorg. Chem 2008, 47, 3358–3365. [Google Scholar]
- Kachi-Terajima, C.; Ishii, M.; Saito, T.; Kanadani, C.; Harada, T.; Kuroda, R. Homochiral 1D helical chain based on an achiral Cu(II) complex. Inorg. Chem 2012, 51, 7502–7507. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar]
- Cudziło, S.; Trzciński, W.; Nita, M.; Michalik, S.; Krompiec, S.; Kruszyński, R.; Kusz, J. Preparation, crystal structure and explosive properties of copper(II) perchlorate complex with 4-amino-1,2,4-triazole and water. Propellants Explos. Pyrotech 2011, 36, 151–159. [Google Scholar]
- Talawar, M.B.; Divekar, C.N.; Makashir, P.S.; Asthana, S.N. Tetrakis-(4-amino-1,2,4-triazole) copper perchlorate: A novel ballistic modifier for composite propellants. J. Propuls. Power 2005, 21, 186–189. [Google Scholar]









| Complex | d(Cu···Cu) (Å) | ∠(Cu–N–N–Cu) a (°) | g | J (cm−1) | P (%) | χTIP × 106 (cm3 mol−1) | zJ′/kB (cm−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| [Cu(μ2-Htrz)(μ2-Cl)2] | 3.405 | −4.67 | 2.013 | −12.4 | - | - | - | [26,32] |
| [Cu(μ2-NH2trz) (μ2-Cl)2] | 3.4895(5), 3.5210(5) | −5.8(3), 8.2(4) | 2.26 | −20.4 | - | - | - | [10,32] |
| [Cu(μ2-NH2trz)2Cl] Cl·H2O (III) | 3.653(2) | 5.2(3), 11.7(2) | 2.13 | −128.4 | 0.65 | 390 | 11(5) | This work |
| [Cu(μ2-NH2trz)2Cl] (SiF6)0.5 ·1.5H2O (IV) | 3.599(2), 3.611(2) | −1.5(5), −13.9(4); −0.6(5), 11.5(4) | 2.13 | −143.0 | 1.02 | 270 | 80(4) | This work |
| 2.13 | −139.9 | 0.90 | 350 | 0 | ||||
| [Cu(μ2-NH2trz)2 (μ2-N3 -N,N)]NO3 | 3.5035(8) | −7.8(4), −13.0(4) | 2.22(1) | −17.7 | - | - | - | [11] |
| [Cu(μ2-NH2trz)2 (μ2-NCS-N)] ClO4 ·0.5H2O | 3.470(2) | 1.5(6), 2.0(5) | 2.21 | −51 | - | - | - | [12] |
| [Cu(μ2-NH2trz)2 (μ2-NO3)]NO3 | 3.5301(5) | −11.4(3), −13.8(3) | 2.12 | −75.1 | - | - | −1.1 | [11] |
| [Cu(μ2-hyetrz)3] (ClO4)2 ·3H2O b | 3.829(2), 3.853(2) | −6.4(7), −10.7(9), −37.5(7); −3.0(7), −11.5(8), −38.0(6) | 2.03(1) | −1.18(2) | - | - | - | [27] |
| [Cu(μ2-hyetrz)3] (CF3SO3)2 ·H2O b | 3.8842(4), 3.9354(4) | −3.3(2), −16.3(2), −28.98(19); −11.3(2), −13.8(2), −20.2(2) | 2.20(1) | 1.45(3) | - | - | - | [29] |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dîrtu, M.M.; Boland, Y.; Gillard, D.; Tinant, B.; Robeyns, K.; Safin, D.A.; Devlin, E.; Sanakis, Y.; Garcia, Y. New Mononuclear Cu(II) Complexes and 1D Chains with 4-Amino-4H-1,2,4-triazole. Int. J. Mol. Sci. 2013, 14, 23597-23613. https://doi.org/10.3390/ijms141223597
Dîrtu MM, Boland Y, Gillard D, Tinant B, Robeyns K, Safin DA, Devlin E, Sanakis Y, Garcia Y. New Mononuclear Cu(II) Complexes and 1D Chains with 4-Amino-4H-1,2,4-triazole. International Journal of Molecular Sciences. 2013; 14(12):23597-23613. https://doi.org/10.3390/ijms141223597
Chicago/Turabian StyleDîrtu, Marinela M., Yves Boland, Damien Gillard, Bernard Tinant, Koen Robeyns, Damir A. Safin, Eamonn Devlin, Yiannis Sanakis, and Yann Garcia. 2013. "New Mononuclear Cu(II) Complexes and 1D Chains with 4-Amino-4H-1,2,4-triazole" International Journal of Molecular Sciences 14, no. 12: 23597-23613. https://doi.org/10.3390/ijms141223597
APA StyleDîrtu, M. M., Boland, Y., Gillard, D., Tinant, B., Robeyns, K., Safin, D. A., Devlin, E., Sanakis, Y., & Garcia, Y. (2013). New Mononuclear Cu(II) Complexes and 1D Chains with 4-Amino-4H-1,2,4-triazole. International Journal of Molecular Sciences, 14(12), 23597-23613. https://doi.org/10.3390/ijms141223597