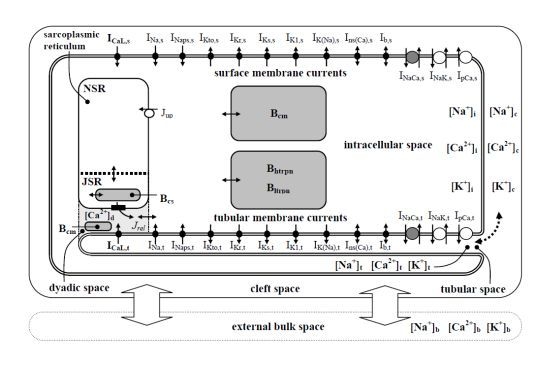
Effect of Ion Concentration Changes in the Limited Extracellular Spaces on Sarcolemmal Ion Transport and Ca2+ Turnover in a Model of Human Ventricular Cardiomyocyte
Abstract
:1. Introduction
2. Results
2.1. Basic Behavior of the Model
2.2. Effect of Ion Concentration Changes in Extracellular Spaces on Intracellular Ca2+ SR Load and Ca2+-Transient at Steady-State Stimulation
2.3. Model Responses to Sudden Changes of Stimulation Rate
3. Discussion
3.1. Validation of the Model
3.2. Conditions of Model Stability
3.3. The Effects of Extracellular Ion Concentration Changes Under Steady-State and Non-Steady-State Conditions
3.4. Cardiomyopathies Alter the Function and Structure of Tubular System
3.5. Limitations of the Study
3.5.1. Inhomogeneities Along the T-Tubules
3.5.2. Deformation of the T-Tubules
3.5.3. Cellular Subtypes and Regional Variations
4. Methods
5. Conclusions
Supplementary Information
ijms-14-24271-s001.pdfAcknowledgments
Appendix
| Symbol | Definition |
|---|---|
| Abbreviations used in the text | |
| AP | Action potential |
| APD | Action potential duration |
| EC | Excitation-contraction |
| SR | Sarcoplasmic reticulum |
| NSR | Network compartment of SR |
| JSR | Junctional compartment of SR |
| Variables used in the text | |
| Vm | Membrane voltage |
| INa | Fast Na+ current |
| ICaL | L-type Ca2+ current |
| INaps | Persistent Na+ current |
| IKto | Transient outward K+ current |
| IKr | Rapid delayed rectifier K+ current |
| IKs | Slow delayed rectifier K+ current |
| IK1 | Inward rectifying K+ current |
| IK(Na) | Sodium-activated K+ current |
| Ins(Ca) | Calcium-activated non-specific current |
| Ib | Background current |
| INaCa | Na+-Ca2+ exchange current |
| INaK | Na+-K+ pump current |
| IpCa | Ca2+ pump current |
| Ix,s | Surface membrane component of current x |
| Ix,t | Tubular membrane component of current x |
| Jup | Ca2+ flow via SR Ca2+ pump |
| Jx,net,t | Net flux of ion x through t-tubular membrane |
| Jx,net,s | Net flux of ion x through surface membrane |
| nx,net,t | Net amount of ion x transferred via t-tubular membrane during a cycle |
| nx,net,s | Net amount of ion x transferred via surface membrane during a cycle |
| [x]b | Free concentration of substance x in external bulk space |
| [x]c | Free concentration of substance x in cleft space |
| [x]t | Free concentration of substance x in t-tubular space |
| [x]d | Free concentration of substance x in dyadic space |
| [x]i | Free concentration of substance x in cytosol |
| [x]end | Free concentration of substance x at the end of cycle |
| [x]mean | Mean free concentration of substance x during a cycle |
| [x]NSR | Free concentration of substance x in NSR |
| [x]JSR | Free concentration of substance x in JSR |
| Parameters/constants used in the text | |
| fi,t | Fraction of ion transporters i in t-tubular membrane |
| τx,ct | Time constant of exchange of ion x between intercellular clefts and t-tubules |
Conflicts of Interest
References
- Courtemanche, M.; Ramirez, R.J.; Nattel, S. Ionic mechanisms underlying human atrial action potential properties: Insight from a mathematical model. Am. J. Physiol 1998, 275, 301–321. [Google Scholar]
- Nygren, A.; Fiset, C.; Firek, L.; Clark, J.W.; Lindblad, D.S.; Clark, R.B.; Giles, W.R. Mathematical model of an adult human atrial cell: the role of K+ currents in repolarization. Circ. Res 1998, 82, 63–81. [Google Scholar]
- Priebe, L.; Beuckelmann, D.J. Simulation study of cellular electric properties in heart failure. Circ. Res 1998, 82, 1206–1223. [Google Scholar]
- Ten Tusscher, K.H.W.J.; Noble, D.; Noble, P.J.; Panfilov, A.V. A model for human ventricular tissue. Am. J. Physiol 2004, 286, 1573–1589. [Google Scholar]
- Iyer, V.; Mazhari, R.; Winslow, R.L. A computational model of the human left-ventricular epicardial myocyte. Biophys. J 2004, 87, 1507–1525. [Google Scholar]
- Fink, M.; Noble, D.; Virag, L.; Varro, A.; Giles, W.R. Contributions of HERG K+ current to repolarization of the human ventricular action potential. Prog. Biophys. Mol. Biol 2008, 96, 357–376. [Google Scholar]
- Grandi, E.; Pasqualini, F.S.; Bers, D.M. A novel computational model of the human ventricular action potential and Ca transient. J. Mol. Cell. Cardiol 2010, 48, 112–121. [Google Scholar]
- O’Hara, T.; Virág, L.; Varró, A.; Rudy, Y. Simulation of the undiseased human cardiac ventricular action potential: Model formulation and experimental validation. PLoS Comput. Biol 2011, 7, e1002061. [Google Scholar]
- Hilgemann, D.W.; Noble, D. Excitation-contraction coupling and extracellular calcium transients in rabbit atrium: Reconstruction of basic cellular mechanisms. Proc. R. Soc. Lond 1987, 230, 163–205. [Google Scholar]
- Hilgemann, D.W. Extracellular calcium transients and action potential configuration changes related to post-stimulatory potentiation in rabbit atrium. J. Gen. Physiol 1986, 87, 675–706. [Google Scholar]
- Hilgemann, D.W. Extracellular calcium transients at single excitations in rabbit atrium measured with tetramethylmurexide. J. Gen. Physiol 1986, 87, 707–735. [Google Scholar]
- Pásek, M.; Christé, G.; Šimurda, J. A quantitative model of the cardiac ventricular cell incorporating the transverse-axial tubular system. Gen. Physiol. Biophys 2003, 22, 355–368. [Google Scholar]
- Pásek, M.; Šimurda, J.; Christé, G. The functional role of cardiac T-tubules explored in a model of rat ventricular myocytes. Philos. Trans. R. Soc. A 2006, 364, 1187–1206. [Google Scholar]
- Pásek, M.; Šimurda, J.; Orchard, C.H.; Christé, G. A model of the guinea-pig ventricular cardiomyocyte incorporating a transverse-axial tubular system. Prog. Biophys. Mol. Biol 2008, 96, 258–280. [Google Scholar]
- Ohler, A.; Weisser-Thomas, J.; Piacentino, V.; Houser, S.R.; Tomaselli, G.F.; O’Rourke, B. Two-photon laser scanning microscopy of the transverse-axial tubule system in ventricular cardiomyocytes from failing and non-failing human hearts. Cardiol. Res. Pract 2009. [Google Scholar] [CrossRef]
- Pásek, M.; Šimurda, J.; Orchard, C.H. Role of t-tubules in the control of trans-sarcolemmal ion flux and intracellular Ca2+ in a model of the rat cardiac ventricular myocyte. Eur. Biophys. J 2012, 41, 491–503. [Google Scholar]
- Pásek, M.; Šimurda, J.; Christé, G.; Orchard, C.H. Modelling the cardiac transverse–axial tubular system. Prog. Biophys. Mol. Biol 2008, 96, 226–243. [Google Scholar]
- Shepherd, N.; McDonough, H.B. Ionic diffusion in transverse tubules of cardiac ventricular myocytes. Am. J. Physiol 1998, 275, 852–860. [Google Scholar]
- Brette, F.; Salle, L.; Orchard, C.H. Differential modulation of L-type Ca2+ current by SR Ca2+ release at the T-tubules and surface membrane of rat ventricular myocytes. Circ. Res 2004, 95, 1–7. [Google Scholar]
- Kunze, D.L. Rate-dependent changes in extracellular potassium in the rabbit atrium. Circ. Res 1977, 41, 122–127. [Google Scholar]
- Attwell, D.; Cohen, I.; Eisner, D.A. The effects of heart rate on the action potential of guinea-pig and human ventricular muscle. J. Physiol 1981, 313, 439–461. [Google Scholar]
- Hilgemann, D.W.; Langer, G.A. Transsarcolemmal calcium movements in arterially perfused rabbit right ventricle measured with extracellular calcium-sensitive dyes. Circ. Res 1984, 54, 461–467. [Google Scholar]
- Almers, W. Potassium concentration changes in the transverse tubules of vertebrate skeletal muscle. Fed. Proc 1980, 39, 1527–1532. [Google Scholar]
- Almers, W.; Fink, R.; Palade, P.T. Calcium depletion in frog muscle tubules: The decline of calcium current under maintained depolarization. J. Physiol 1981, 312, 177–207. [Google Scholar]
- Li, G.R.; Feng, J.; Yue, L.; Carrier, M. Transmural heterogeneity of action potentials and Ito1 in myocytes isolated from the human right ventricle. Am. J. Physiol 1998, 275, 369–377. [Google Scholar]
- Li, G.R.; Yang, B.; Feng, J.; Bosch, R.F.; Carrier, M.; Nattel, S. Transmembrane ICa contributes to rate-dependent changes of action potentials in human ventricular myocytes. Am. J. Physiol 1999, 276, 98–106. [Google Scholar]
- Schmidt, U.; Hajjar, R.J.; Helm, P.A.; Kim, C.S.; Doye, A.A.; Gwathmey, J.A. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J. Mol. Cell. Cardiol 1998, 30, 1929–1937. [Google Scholar]
- Pieske, B.; Maier, L.S.; Piacentino, V.; Weisser, J.; Hasenfuss, G.; Houser, S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation 2002, 106, 447–453. [Google Scholar]
- Beuckelmann, D.J.; Näbauer, M.; Erdmann, E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation 1992, 88, 1046–1055. [Google Scholar]
- Pieske, B.; Houser, S.R. [Na+]i handling in the failing human heart. Cardiovasc. Res 2003, 57, 874–886. [Google Scholar]
- Brette, F.; Orchard, C.H. T-tubule function in mammalian cardiac myocytes. Circ. Res 2003, 92, 1182–1192. [Google Scholar]
- Orchard, C.H.; Pásek, M.; Brette, F. The role of mammalian cardiac t-tubules in excitation-contraction coupling: Experimental and computational approaches. Exp. Physiol 2009, 94, 509–519. [Google Scholar]
- Wei, S.; Guo, A.; Chen, B.; Kutschke, W.; Xie, Y.P.; Zimmerman, K.; Weiss, R.M.; Anderson, M.E.; Cheng, H.; Song, L.S. T-tubule remodeling during transition from hypertrophy to heart failure. Circ. Res 2010, 107, 520–531. [Google Scholar]
- He, J.; Conklin, M.W.; Foell, J.D.; Wolff, M.R.; Haworth, R.A.; Coronado, R.; Kamp, T.J. Reduction in density of transverse tubules and L-type Ca2+ channels in canine tachycardia induced heart failure. Cardiovasc. Res 2001, 49, 298–307. [Google Scholar]
- Louch, W.E.; Bito, V.; Heinzel, F.R.; Macianskiene, R.; Vanhaecke, J.; Flameng, W.; Mubagwa, K.; Sipido, K.R. Reduced synchrony of Ca2+ release with loss of T-tubules—A comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc. Res 2004, 62, 63–73. [Google Scholar]
- Kaprielian, R.R.; Stevenson, S.; Rothery, S.M.; Cullen, M.J.; Severs, N.J. Distinct patterns of dystrophin organization in myocyte sarcolemma and transverse tubules of normal and diseased human myocardium. Circulation 2000, 101, 2586–2594. [Google Scholar]
- Wong, C.; Soeller, C.; Burton, L.; Cannell, M. Changes in transverse-tubular system architecture in myocytes from diseased human ventricles. Biophys. J 2001, 80, 588A. [Google Scholar]
- Lyon, A.R.; MacLeod, K.T.; Zhang, Y.; Garcia, E.; Kanda, G.K.; Lab, M.J.; Korchev, Y.E.; Harding, S.E.; Gorelik, J. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc. Natl. Acad. Sci. USA 2009, 106, 6854–6859. [Google Scholar]
- Ferrantini, C.; Crocini, C.; Coppini, R.; Vanzi, F.; Tesi, C.; Cerbai, E.; Poggesi, C.; Pavone, F.S.; Sacconi, L. The transverse-axial tubular system of cardiomyocytes. Cell. Mol. Life Sci 2013, 70, 4695–4710. [Google Scholar]
- Cannell, M.B.; Crossman, D.J.; Soeller, C. Effect of changes in action potential spike configuration, junctional sarcoplasmic reticulum micro-architecture and altered t-tubule structure in human heart failure. J. Muscle Res. Cell. Motil 2006, 27, 297–306. [Google Scholar]
- Maron, B.J.; Ferrans, V.J.; Roberts, W.C. Ultrastructural features of degenerated cardiac muscle cells in patients with cardiac hypertrophy. Am. J. Pathol 1975, 79, 387–434. [Google Scholar]
- Balijepalli, R.C.; Lokuta, A.J.; Maertz, N.A.; Buck, J.M.; Haworth, R.A.; Valdivia, H.H.; Kamp, T.J. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc. Res 2003, 59, 67–77. [Google Scholar]
- Prinz, C.; Farr, M.; Laser, K.T.; Esdorn, H.; Piper, C.; Horstkotte, D.; Faber, L. Determining the role of fibrosis in hypertrophic cardiomyopathy. Expert Rev. Cardiovasc Ther 2013, 11, 495–504. [Google Scholar]
- Franzini–Armstrong, C.; Protasi, F.; Tijskens, P. The assembly of calcium release units in cardiac muscle. Ann. N.Y. Acad. Sci 2005, 1047, 76–85. [Google Scholar]
- Šimurda, J.; Pásek, M.; Christé, G.; Šimurdova, M. Modelling the distribution of [Ca2+] within the cardiac T–tubule—Effects of Ca2+ current distribution and changes in extracellular [Ca2+] (Abstract). J. Physiol 2004, 561, PC5. [Google Scholar]
- Dyachenko, V.; Christ, A.; Gubanov, R.; Isenberg, G. Bending of z–lines by mechanical stimuli: An input signal for integrin dependent modulation of ion channels? Prog. Biophys. Mol. Biol 2008, 97, 196–216. [Google Scholar]
- McNary, T.G.; Spitzer, K.W.; Holloway, H.; Bridge, J.H.; Kohl, P.; Sachse, F.B. Mechanical modulation of the transverse tubular system of ventricular cardiomyocytes. Prog. Biophys. Mol. Biol 2012, 110, 218–225. [Google Scholar]
- Tidball, J.G.; Smith, R.; Shattock, M.J.; Bers, D.M. Differences in action potential configuration in ventricular trabeculae correlate with differences in density of transverse tubule–sarcoplasmic reticulum couplings. J. Mol. Cell. Cardiol 1988, 20, 539–546. [Google Scholar]
- Tidball, J.G.; Cederdahl, J.E.; Bers, D.M. Quantitative analysis of regional variability in the distribution of transverse tubules in rabbit myocardium. Cell Tissue Res 1991, 264, 293–298. [Google Scholar]
- Fowler, M.R.; Dobson, R.S.; Orchard, C.H.; Harrison, S.M. Functional consequences of detubulation of isolated rat ventricular myocytes. Cardiovasc. Res 2004, 62, 529–537. [Google Scholar]











© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hrabcová, D.; Pásek, M.; Šimurda, J.; Christé, G. Effect of Ion Concentration Changes in the Limited Extracellular Spaces on Sarcolemmal Ion Transport and Ca2+ Turnover in a Model of Human Ventricular Cardiomyocyte. Int. J. Mol. Sci. 2013, 14, 24271-24292. https://doi.org/10.3390/ijms141224271
Hrabcová D, Pásek M, Šimurda J, Christé G. Effect of Ion Concentration Changes in the Limited Extracellular Spaces on Sarcolemmal Ion Transport and Ca2+ Turnover in a Model of Human Ventricular Cardiomyocyte. International Journal of Molecular Sciences. 2013; 14(12):24271-24292. https://doi.org/10.3390/ijms141224271
Chicago/Turabian StyleHrabcová, Dana, Michal Pásek, Jiří Šimurda, and Georges Christé. 2013. "Effect of Ion Concentration Changes in the Limited Extracellular Spaces on Sarcolemmal Ion Transport and Ca2+ Turnover in a Model of Human Ventricular Cardiomyocyte" International Journal of Molecular Sciences 14, no. 12: 24271-24292. https://doi.org/10.3390/ijms141224271
APA StyleHrabcová, D., Pásek, M., Šimurda, J., & Christé, G. (2013). Effect of Ion Concentration Changes in the Limited Extracellular Spaces on Sarcolemmal Ion Transport and Ca2+ Turnover in a Model of Human Ventricular Cardiomyocyte. International Journal of Molecular Sciences, 14(12), 24271-24292. https://doi.org/10.3390/ijms141224271