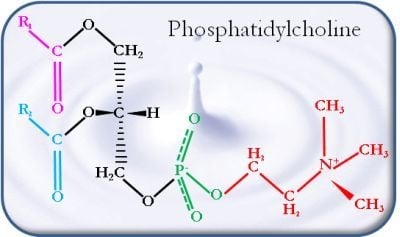
Phospholipids in Milk Fat: Composition, Biological and Technological Significance, and Analytical Strategies
Abstract
:1. Introduction
2. Nature and Characteristics of Phospholipids in Dairy Products
3. Biological Activity and Health Effects
3.1. Cardiovascular Diseases
3.2. Inflammation and Gastrointestinal Infections
3.3. Stress Conditions
3.4. Cancer
3.5. Cholesterol Absorption
3.6. Nervous System Myelination and Neurological Development
4. Technological Properties
5. Analytical Strategies for PL Determination
5.1. Extraction of Fat from Milk and Dairy Products
5.2. PL Separation from Lipid Matrix
5.3. Quantification and Identification of Single PLs
5.3.1. HPLC Coupled with ELSD or MS
5.3.2. NMR Technique
5.4. Determination of the FAs Bonded to PL Molecules
6. Conclusions
Abbreviations
| DHSM | dihydrosphingomyelin |
| ELSD | evaporative light scattering detector |
| ePC | ether phosphatidylcholine |
| ePE | ether phosphatidylethanolamine |
| EPLAS | phosphatidylethanolamine plasmalogen |
| FA | fatty acid |
| GluCer | glucosylceramide |
| LacCer | lactosylceramide |
| LDL | low density lipoproteins: HDL, high density lipoproteins |
| HPLC | high performance liquid chromatography |
| LPA | lysophosphatidic acid |
| LPC | lysophosphatidylcholine |
| LPE | lysophosphatidylethanolamine |
| LPS | lysophosphatidylserine |
| MFGM | milk fat globule membrane |
| MMPE | monomethylphosphatidylethanolamine |
| MS | mass spectrometer |
| NMR | nuclear magnetic resonance |
| PA | phosphatidic acid |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PE-cer | phosphoethanolamine-ceramide |
| PG | phosphatidylglycerol |
| PI | phosphatidylinositol |
| PL | phospholipid |
| PLA2 | phospholipase A2 |
| PS | phosphatidylserine |
| SM | sphingomyelin |
Conflict of Interest
References
- Singh, H. The milk fat globule membrane—A biophysical system for food applications. Curr. Opin. Colloid Interface Sci 2006, 11, 154–163. [Google Scholar]
- Evers, J.M.; Haverkamp, R.G.; Holroyd, S.E.; Jameson, G.B.; Mackenzie, D.D.S.; McCarthy, O.J. Heterogeneity of milk fat globule membrane structure and composition as observed using fluorescence microscopy techniques. Int. Dairy J 2008, 18, 1081–1089. [Google Scholar]
- Gallier, S.; Gragson, D.; Cabral, C.; Jimenez-Flores, R.; Everett, D.W. Using confocal laser scanning microscopy to probe the milk fat globule membrane and associated proteins. J. Agric. Food Chem 2010, 58, 4250–4257. [Google Scholar]
- Lopez, C.; Madec, M.N.; Jimenez-Flores, R. Lipid rafts in the bovine milk fat globule membrane revealed by the lateral segregation of phospholipids and heterogeneous distribution of glycoproteins. Food Chem 2010, 120, 22–33. [Google Scholar]
- Spitsberg, V.L. Bovine milk fat globule membrane as a potential nutraceutical. J. Dairy Sci 2005, 88, 2289–2294. [Google Scholar]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; van Camp, J. Nutritional and technological aspects of milk fat globule membrane material. Int. Dairy J 2008, 18, 436–457. [Google Scholar]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis 2012, 11, 1–16. [Google Scholar]
- Kanno, C. Emulsifying properties of bovine milk fat globule membrane in milk fat emulsion: Conditions for the reconstitution of milk fat globules. J. Food Sci 1989, 54, 1534–1539. [Google Scholar]
- Beare-Rogers, J.; Dieffenbacher, A.; Holm, J.V. Lexicon of lipid nutrition. (IUPAC Technical Report). Pure Appl. Chem 2001, 73, 685–744. [Google Scholar]
- Hay, J.D.; Morrison, W.R. Polar lipids in bovine milk. III. Isomeric cis and trans monoenoic and dienoic fatty acids, and alkyl and alkenyl ethers in phopshatidylcholine and phopshatidylethanolammine. Biochim. Biophys. Acta 1971, 248, 71–79. [Google Scholar]
- Murgia, S.; Mele, S.; Monduzzi, M. Quantitative characterization of phospholipids in milk fat via 31P NMR using a monophasic solvent mixture. Lipids 2003, 38, 585–591. [Google Scholar]
- Gallier, S.; Gragson, D.; Cabral, C.; Jimenez-Flores, R.; Everett, D.W. Composition and fatty acid distribution of bovine milk phospholipids from processed milk products. J. Agric. Food Chem 2010, 58, 10503–10511. [Google Scholar]
- Garcia, C.; Lutz, N.W.; Confort-Gouny, S.; Cozzone, P.J.; Armand, M.; Bernard, M. Phospholipid fingerprints of milk from different mammalians determined by 31P NMR: Towards specific interest in human health. Food Chem 2012, 135, 1777–1783. [Google Scholar]
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and functions. Prog. Lipid Res 2001, 40, 199–229. [Google Scholar]
- Christie, W.W. Lipid library. Available online: http://www.lipidlibrary.aocs.org accessed on 8 December 2012.
- Deeth, H.C. The role of phospholipids in the stability of milk fat globules. Aust. J. Dairy Technol 1997, 52, 44–46. [Google Scholar]
- Fong, B.Y.; Norris, C.S.; MacGibbon, A.K.H. Protein and lipid composition of bovine milk-fat globule membrane. Int. Dairy J 2007, 17, 275–288. [Google Scholar]
- Graves, E.L.F.; Beaulieu, A.D.; Drackley, J.K. Factors affecting the concentration of sphingomyelin in bovine milk. J. Dairy Sci 2007, 90, 706–715. [Google Scholar]
- Lopez, C.; Briard-Bion, V.; Menard, O.; Rousseau, F.; Pradel, P.; Besle, J.-M. Phospholipid, sphingolipid, and fatty acid composition of the milk fat globule membrane are modified by diet. J. Agric. Food Chem 2008, 56, 5226–5236. [Google Scholar]
- Bitman, J.; Wood, D.L. Changes in milk fat phospholipids during lactation. J. Dairy Sci 1990, 73, 1208–1216. [Google Scholar]
- Fagan, P.; Wijesundera, C. Liquid chromatographic analysis of milk phospholipids with on-line pre-concentration. J. Chromatogr. A 2004, 1054, 241–249. [Google Scholar]
- Avalli, A.; Contarini, G. Determination of phospholipids in dairy products by SPE/HPLC/ELSD. J. Chromatogr. A 2005, 1071, 185–190. [Google Scholar]
- Andreotti, G.; Trivellone, E.; Motta, A. Characterization of buffalo milk by 31P-nuclear magnetic resonance spectroscopy. J. Food Compos. Anal 2006, 19, 843–849. [Google Scholar]
- Rombaut, R.; van Camp, J.; Dewettinck, K. Phospho- and sphingolipid distribution during processing of milk, butter and whey. Int. J. Food Sci. Technol 2006, 41, 435–443. [Google Scholar]
- Rombaut, R.; Dewettinck, K.; van Camp, J. Phospho- and sphingolipid content of selected dairy products as determined by HPLC coupled to an evaporative light scattering detector (HPLC–ELSD). J. Food Compos. Anal 2007, 20, 308–312. [Google Scholar]
- Fauquant, C.; Briard-Bion, V.; Leconte, N.; Guichardant, M.; Michalski, M.C. Membrane phospholipids and sterols in microfiltered milk fat globules. Eur. J. Lipid Sci. Technol 2007, 109, 1167–1173. [Google Scholar]
- Sanchez-Juanes, F.; Alonso, J.M.; Zancada, L.; Hueso, P. Distribution and fatty acid content of phospholipids from bovine milk and bovine milk membranes. Int. Dairy J 2009, 19, 273–278. [Google Scholar]
- Rodríguez-Alcalá, L.M.; Fontecha, J. Major lipid classes separation of buttermilk, and cows, goats and ewes milk by high performance liquid chromatography with an evaporative light scattering detector focused on the phospholipid fraction. J. Chromatogr. A 2010, 1217, 3063–3066. [Google Scholar]
- Le, T.T.; Miocinovic, J.; Nguyen, T.M.; Rombaut, R.; van Camp, J.; Dewettinck, K. Improved solvent extraction procedure and high-performance liquid chromatography evaporative light-scattering detector method for analysis of polar lipids from dairy materials. J. Agric. Food Chem 2011, 59, 10407–10413. [Google Scholar]
- Donato, P.; Cacciola, F.; Cichello, F.; Russo, M.; Dugo, P.; Mondello, L. Determination of phospholipids in milk samples by means of hydrophilic interaction liquid chromatography coupled to evaporative light scattering and mass spectrometry detection. J. Chromatogr. A 2011, 1218, 6476–6482. [Google Scholar]
- Kielbowicz, G.; Micek, P.; Wawrzenczyk, C. A new liquid chromatography method with charge aerosol detector (CAD) for the determination of phospholipid classes. Application to milk phospholipids. Talanta 2013, 105, 28–33. [Google Scholar]
- Benoit, B.; Fauquant, C.; Daira, P.; Peretti, N.; Guichardant, M.; Michalski, M.-C. Phospholipid species and minor sterols in French human milks. Food Chem 2010, 120, 684–691. [Google Scholar]
- Lamothe, S.; Robitaille, G.; St-Gelais, D.; Britten, M. Butter making from caprine creams: Effect of washing treatment on phospholipids and milk fat globule membrane proteins distribution. J. Dairy Res 2008, 75, 439–443. [Google Scholar]
- MacKenzie, A.; Vyssotski, M.; Nekrasov, E. Quantitative analysis of dairy phospholipids by 31P NMR. J. Am. Oil Chem. Soc 2009, 86, 757–763. [Google Scholar]
- Costa, M.R.; Elias-Argote, X.E.; Jiménez-Flores, R.; Gigante, M.L. Use of ultrafiltration and supercritical fluid extraction to obtain a whey buttermilk powder enriched in milk fat globule membrane phospholipids. Int. Dairy J 2010, 20, 598–602. [Google Scholar]
- Danielsen, E.M.; Hansen, G.H. Lipid raft organization and function in brush borders of epithelial cells. Mol. Membr. Biol 2006, 23, 71–79. [Google Scholar]
- Vesper, H.; Schmelz, E.-M.; Nikolova-Karakashian, M.N.; Dillehay, D.L.; Lynch, D.V.; Merrill, A.H. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J. Nutr 1999, 129, 1239–1250. [Google Scholar]
- Duan, R.-D.; Nilsson, A. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog. Lipid Res 2009, 48, 62–72. [Google Scholar]
- Cohn, J.S.; Kamili, A.; Wat, E.; Chung, R.W.S.; Tandy, S. Dietary phospholipids and intestinal cholesterol absorption. Nutrients 2010, 2, 116–127. [Google Scholar]
- Pereira, M.A.; Jacobs, D.R.; van Horn, L.; Slattery, M.L.; Kartashov, A.I.; Ludwig, D.S. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: The CARDIA study. JAMA 2002, 287, 2081–2089. [Google Scholar]
- Choi, H.K.; Willett, W.C.; Stampfer, M.J.; Rimm, E.; Hu, F.B. Dairy consumption and risk of type 2 diabetes mellitus in men: A prospective study. Arch. Intern. Med 2005, 165, 997–1003. [Google Scholar]
- Wat, E.; Tandy, S.; Kaper, E.; Kamili, A.; Chung, R.W.S.; Brown, A.; Rowney, M.; Cohn, J.S. Dietary phospholipid-rich dairy milk extract reduces hepatomegaly, hepatic steatosis and hyperlipidemia in mice fed a high-fat diet. Atherosclerosis 2009, 205, 144–150. [Google Scholar]
- Watanabe, S.; Takahashi, T.; Tanaka, L.; Haruta, Y.; Shiota, M.; Hosokawa, M.; Miyashita, K. The effect of milk polar lipids separated from butter serum on the lipid levels in the liver and the plasma of obese-model mouse (KK-Ay). J. Funct. Foods 2011, 3, 313–320. [Google Scholar]
- Ohlsson, L.; Burling, H.; Duan, R.-D.; Nilsson, A. Effects of a sphingolipid-enriched dairy formulation on postprandial lipid concentrations. Eur. J. Clin. Nutr 2010, 64, 1344–1349. [Google Scholar]
- Ohlsson, L.; Burling, H.; Nilsson, A. Long term effects on human plasma lipoproteins of a formulation entiched in butter milk polar lipid. Lipids Health Dis 2009, 8, 44. [Google Scholar]
- Keller, S.; Malarski, A.; Reuther, C.; Kertscher, R.; Kiehntopf, M.; Jahreis, G. Milk phospholipid and plant sterol-dependent modulation of plasma lipids in healthy volunteers. Eur. J. Nutr. 2012. [Google Scholar] [CrossRef]
- Mallat, Z.; Lambeau, G.; Tedgui, A. Lipoprotein-associated and secreted phospholipases a2 in cardiovascular disease: Roles as biological effectors and biomarkers. Circulation 2010, 122, 2183–2200. [Google Scholar]
- Lambeau, G.; Gelb, M.H. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem 2008, 77, 495–520. [Google Scholar]
- Hui, D.Y. Phospholipase A2 enzymes in metabolic and cardiovascular diseases. Curr. Opin. Lipidol 2012, 23, 235–240. [Google Scholar]
- Kuchta, A.M.; Kelly, P.M.; Stanton, C.; Devery, R.A. Milk fat globule membrane—A source of polar lipids for colon health? A review. Int. J. Dairy Technol 2012, 65, 315–333. [Google Scholar]
- Veereman-Wauters, G.; Staelens, S.; Rombaut, R.; Dewettinck, K.; Deboutte, D.; Brummer, R.-J.; Boone, M.; Le Ruyet, P. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 2012, 28, 749–752. [Google Scholar]
- Dial, E.J.; Lichtenberg, L.M. A role for milk phospholipids in protection against gastric acid. Studies in adult and suckling rats. Gastroenterology 1984, 87, 379–385. [Google Scholar]
- Anand, B.S.; Romero, J.J.; Sanduja, S.K.; Lichtenberger, L.M. Phospholipid association reduces the gastric mucosal toxicity of aspirin in human subjects. Am. J. Gastroenterol 1999, 94, 1818–1822. [Google Scholar]
- Sprong, C.; Hulstein, M.; van der Meer, R. Phospholipid rich butter milk decreases the gastro-intestinal survival and translocation of listeria in rats. Gastroenterology 1998, 114, A1090. [Google Scholar]
- Sprong, R.C.; Hulstein, M.F.E.; van der Meer, R. Bactericidal activities of milk lipids. Antimicrob. Agents Ch 2001, 45, 1298–1301. [Google Scholar]
- Sprong, R.C.; Hulstein, M.F.E.; van der Meer, R. Bovine milk fat components inhibit food-borne pathogens. Int. Dairy J 2002, 12, 209–215. [Google Scholar]
- Hartmann, P.; Szabó, A.; Erős, G.; Gurabi, D.; Horváth, G.; Németh, I.; Ghyczy, M.; Boros, M. Anti-inflammatory effects of phosphatidylcholine in neutrophil leukocyte-dependent acute arthritis in rats. Eur. J. Pharmacol 2009, 622, 58–64. [Google Scholar]
- Erős, G.; Ibrahim, S.; Siebert, N.; Boros, M.; Vollmar, B. Oral phosphatidylcholine pretreatment alleviates the signs of experimental rheumatoid arthritis. Arthritis Res. Ther 2009, 11, R43. [Google Scholar]
- Hellhammer, J.; Fries, E.; Buss, C.; Engert, V.; Tuch, A.; Rutenberg, D.; Hellhammer, D. Effects of soy lecithin phosphatidic acid and phosphatidylserine complex (PAS) on the endocrine and psychological responses to mental stress. Stress 2004, 7, 119–126. [Google Scholar]
- Schubert, M.; Contreras, C.; Franz, N.; Hellhammer, J. Milk-based phospholipids increase morning cortisol availability and improve memory in chronically stressed men. Nutr. Res 2011, 31, 413–420. [Google Scholar]
- Hellhammer, J.; Waladkhani, A.R.; Hero, T.; Buss, C. Effects of milk phospholipid on memory and psychological stress response. Br. Food J 2010, 112, 1124–1137. [Google Scholar]
- Lemonnier, L.A.; Dillehay, D.L.; Vespremi, M.J.; Abrams, J.; Brody, E.; Schmelz, E.M. Sphingomyelin in the suppression of colon tumors: Prevention versus intervention. Arch. Biochem. Biophys 2003, 419, 129–138. [Google Scholar]
- Russell, A.; Laubscher, A.; Jimenez-Flores, R.; Laiho, L.H. Investigating the protective properties of milk phospholipids against ultraviolet light exposure in a skin equivalent model. Proc. SPIE 2010, 7569, 75692Z:1–75692Z:9. [Google Scholar]
- Nilsson, A.; Duan, R.-D. Absorption and lipoprotein transport of sphingomielin. J. Lipid Res 2006, 47, 154–171. [Google Scholar]
- Kamili, A.; Wat, E.; Chung, R.W.S.; Tandy, S.; Weir, J.M.; Meikle, P.J.; Cohn, J.S. Hepatic accumulation of intestinal cholesterol is decreased and fecal cholesterol excretion is increased in mice fed a high-fat diet supplemented with milk phospholipids. Nutr. Metab 2010, 7, 90–101. [Google Scholar]
- Harder, T.; Simons, K. Caveolae, DIGs, and the dynamics of sphingolipid cholesterol microdomains. Curr. Opin. Cell Biol 1997, 9, 534–542. [Google Scholar]
- Noh, S.K.; Koo, S.I. Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. J. Nutr 2004, 134, 2611–2616. [Google Scholar]
- Kinney, H.C.; Brody, B.A.; Kloman, A.S.; Gilles, F.H. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J. Neuropath. Exp. Neur 1988, 47, 217–234. [Google Scholar]
- Oshida, K.; Shimizu, T.; Takase, M.; Tamura, Y.; Shimizu, T.; Yamashiro, Y. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatr. Res 2003, 53, 589–593. [Google Scholar]
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The pilot study: Sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev 2012, 35, 45–52. [Google Scholar]
- Lindholm, D.; Wootz, H.; Korhonen, L. ER stress and neurodegenerative diseases. Cell Death Differ 2006, 13, 385–392. [Google Scholar]
- Nagai, K. Bovine milk phospholipid fraction protects Neuro2a cells from endoplasmic reticulum stress via PKC activation and autophagy. J. Biosci. Bioeng 2012, 114, 466–471. [Google Scholar]
- Vanderghem, C.; Bodson, P.; Danthine, S.; Paquot, M.; Deroanne, C.; Blecker, C. Milk fat globule membrane and buttermilks: From composition to valorization. Biotechnol. Agron. Soc 2010, 14, 485–500. [Google Scholar]
- Sodini, I.; Morin, P.; Olabi, A.; Jimenez-Flores, R. Compositional and functional properties of buttermilk: A comparison between sweet, sour, and whey buttermilk. J. Dairy Sci 2006, 89, 525–536. [Google Scholar]
- Rombaut, R.; Dejonckheere, V.; Dewettinck, K. Microfiltration of butter serum upon casein micelle destabilization. J. Dairy Sci 2006, 89, 1915–1925. [Google Scholar]
- Corredig, M.; Roesch, R.R.; Dalgleish, D.G. Production of a novel ingredient from buttermilk. J. Dairy Sci 2003, 86, 2744–2750. [Google Scholar]
- Phan, T.T.Q.; Asaduzzaman, M.; Le, T.T.; Fredrick, E.; van der Meeren, P.; Dewettinck, K. Composition and emulsifying properties of a milk fat globule membrane enriched material. Int. Dairy J 2013, 29, 99–106. [Google Scholar]
- Kasinos, M.; Le, T.T.; van der Meeren, P. Improved heat stability of recombined evaporated milk emulsions upon addition of phospholipid enriched dairy by-products. Food Hydrocolloids.
- Bezelgues, J.-B.; Morgan, F.; Palomo, G.; Crosset-Perrotin, L.; Ducret, P. Milk fat globule membrane as a potential delivery system for liposoluble nutrients. J. Dairy Sci 2009, 92, 2524–2528. [Google Scholar]
- Gülseren, I.; Guri, A.; Corredig, M. Encapsulation of tea polyphenols in nanoliposomes prepared with milk phospholipids and their effect on the viability of HT-29 human carcinoma cells. Food Dig 2012, 3, 36–45. [Google Scholar]
- Farhang, B.; Kakuda, Y.; Corredig, M. Encapsulation of ascorbic acid in liposomes prepared with milk fat globule membrane-derived phospholipids. Dairy Sci. Technol 2012, 92, 353–366. [Google Scholar]
- ISO 1211, Milk: Determination of Fat Content—Gravimetric Method (Reference method); International Standard ISO: Geneva, Switzerland, 2010.
- ISO 1735, Cheese and processed cheese products: Determination of Fat Content—Gravimetric Method (Reference method); International Standard ISO: Geneva, Switzerland, 2004.
- Duthie, A.H.; Patton, S. New method for extraction of milk phospholipids. J. Dairy Sci 1964, 48, 170–174. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem 1957, 226, 497–509. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys 1959, 37, 911–917. [Google Scholar]
- Shahidi, F.; Wanasundara, P.K.J.P.D. Extraction and Analysis of Lipids. In Food Lipids: Chemistry, Nutrition, and Biotechnology, 3rd ed; Akho, C.C., Min, D.B., Eds.; Francis & Taylor: New York, NY, USA, 2008; pp. 125–156. [Google Scholar]
- Shaikh, N.A. Assessment of various techniques for the quantitative extraction of lysophospholipids from myocardial tissues. Anal. Biochem 1994, 216, 313–321. [Google Scholar]
- Rombaut, R.; Camp, J.V.; Dewettinck, K. Analysis of phospho-and sphingolipids in dairy products by a new hplc method. J. Dairy Sci 2005, 88, 482–488. [Google Scholar]
- Hauff, S.; Vetter, W. Quantification of fatty acids as methyl esters and phospholipids in cheese samples after separation of triacylglycerides and phospholipids. Anal. Chim. Acta 2009, 636, 229–235. [Google Scholar]
- Caboni, M.F.; Menotta, S.; Lercker, G. Separation and analysis of phospholipids in different foods with a light-scattering detector. J. Am. Oil Chem. Soc 1996, 73, 1561–1566. [Google Scholar]
- Godoy Ramos, R.; Libong, D.; Rakotomanga, M.; Gaudin, K.; Loiseau, P.M.; Chaminade, P. Comparison between charged aerosol detection and light scattering detection for the analysis of Leishmania membrane phospholipids. J. Chromatogr. A 2008, 1209, 88–94. [Google Scholar]
- Spyros, A.; Dais, P. Application of 31P NMR spectroscopy in food analysis. Quantitative determination of the mono- and diglyceride composition of olive oils. J. Agric. Food Chem 2000, 48, 802–805. [Google Scholar]
- Christie, W.W. Preparation of Derivatives of Fatty Acids. In Lipid Analysis, 3rd ed; The Oily Press, PJ Barnes & Associates: Bridgewater, UK, 2003; pp. 205–224. [Google Scholar]



| Reference | Polar lipids | PE | PI | PS | PC | SM | Note |
|---|---|---|---|---|---|---|---|
| [21] | 0.69 | 38.6 | - | - | 32.2 | 29.2 | 1 |
| [22] | 0.36 | 32.3 | 9.3 | 10.5 | 27.3 | 20.5 | |
| [23] | 26.9 | 13.7 | 4.1 | 27.5 | 27.7 | ||
| [24] | 0.96 | 33.2 | 5.2 | 9.3 | 27.4 | 25.1 | |
| [25] | 0.7 | 46.4 | 5.3 | 7.4 | 21.1 | 19.8 | |
| [17] | 32.6 | 7.6 | 5.3 | 33.2 | 21.3 | ||
| [26] | 36.4 | 7.6 | 6.5 | 32.1 | 17.3 | ||
| [19] | 0.25–0.30 | 26.8 | 13.6 | 16.1 | 22 | 21.6 | |
| [27] | 0.48 | 28.5 | 14.1 | - | 32.7 | 23 | 1,2 |
| [28] | 0.36 | 38.5 | 6.5 | 7.7 | 25.9 | 21.4 | |
| [12] | 26.4 | 3.4 | 2 | 42.8 | 25.5 | ||
| [29] | 0.69 | 36.9 | 6.1 | 6.3 | 27 | 23.7 | |
| [30] | 72.3 | 1.4 | 11.5 | 8 | 7.9 | ||
| [13] | 33.8 | 3.9 | 10.6 | 30.5 | 21.2 | ||
| [31] | 0.65–0.89 | 34.2 | 7.7 | 8.6 | 45.5 | 4.1 | 1 |
| Reference | Species | PE | PI | PS | PC | SM | Note |
|---|---|---|---|---|---|---|---|
| [23] | buffalo | 24,5 | 19,7 | 6,6 | 24,3 | 24,9 | |
| [28] | goat | 31.7 | 6.3 | 8.3 | 28.5 | 25.2 | |
| ewe | 34.4 | 4.4 | 5.2 | 28.6 | 27.4 | ||
| [32] | human | 21.3 | 16.4 | 19 | 43.3 | 1 | |
| [30] | donkey | 60.2 | 2.4 | 11.2 | 17.3 | 8.8 | |
| [13] | mare | 24.3 | 8.5 | 10.6 | 27.8 | 28.9 | |
| human | 21.7 | 4.5 | 9.6 | 29 | 35.2 | ||
| camel | 34.3 | 4.9 | 10.5 | 22.1 | 28.1 |
| Reference | Matrix | Polar lipids | PE | PI | PS | PC | SM |
|---|---|---|---|---|---|---|---|
| [22] | cream | 0.86 | 42.7 | 6.8 | 7.2 | 14.6 | 28.6 |
| butter | 0.2 | 31 | 11.9 | 15.3 | 24.7 | 17.1 | |
| buttermilk | 4.49 | 33.5 | 2.4 | 10.3 | 35.5 | 18.3 | |
| [33] | cow cream | 0.17 | |||||
| cow buttermilk | 0.17 | 38.7 | 9.3 | 9.1 | 23.9 | 18.9 | |
| cow butter serum | 0.88 | 27.2 | 10.8 | 7.2 | 29.8 | 24.9 | |
| goat cream | 0.2 | ||||||
| goat buttermilk | 0.19 | 35.2 | 9.8 | 9.9 | 24.8 | 20.3 | |
| goat butter serum | 1.01 | 27.1 | 11.7 | 8.2 | 26.2 | 26.8 | |
| [34] | cream | 26.7 | 7.5 | 11.7 | 26.5 | 20.8 | |
| [35] | cream | 5.65 | 17.7 | 15.4 | 11.3 | 33.7 | 21.8 |
| butter | 5.31 | 17.7 | 15.8 | 11.5 | 33.3 | 21.8 | |
| buttermilk | 12.4 | 17 | 7.1 | 8.1 | 46.1 | 21.7 | |
| [12] | buttermilk | 8.4 | 8.2 | 4.6 | 51.2 | 27.6 |
| Reference | Matrix | SPE phase | Solvents for non polar compound elution (v/v) | Solvents for PL elution (v/v) |
|---|---|---|---|---|
| [91] | Egg powder, chicken meat, cheese, salami | Silica | hexane/diethyl-ether (8:2) and (1:1) | methanol and metanol/acetic acid (1% to 5%) |
| Aminopropyl (NH2) | chloroform/isopropanol (2:1) and diethyl-ether/acetic acid (98:2) | methanol | ||
| Octadecyl (C18) | methanol and methanol/chloroform (4:1) | methanol/water (4:1) | ||
| Octyl (C8) (*) | chloroform/methanol (3:2) and chloroform | methanol | ||
| [22] | milk, cream, butter, fresh buttermilk | Silica | hexane/diethyl-ether (8:2) and (1:1) | methanol |
| Silica (*) | hexane/diethyl-ether (8:2) and (1:1) | methanol and chloroform/methanol/water (3:5:2) | ||
| Octyl (C8) | chloroform/methanol (3:2) and chloroform | methanol | ||
| [90] | cheese | Silica with 20% water | cyclohexane/ethyl acetate (1:1) | ethyl acetate/methanol (1:1), methanol and methanol/water (98:2). |
| [12] | milk, cream, powdered buttermilk | Aminopropyl (NH2-bonded) | chloroform/isopropanol (2:1) and diethyl-ether/acetic acid (98:2) | methanol |
| Silica (*) | hexane/diethyl-ether (1:1) | methanol and chloroform/methanol/water (3:5:2) | ||
| Silica | hexane/diethyl-ether (8:2) and (1:1) | methanol and chloroform/methanol/water (3:5:2) | ||
| [30] | milk of different species | Silica | hexane/diethyl-ether (8:2 and 1:1) | methanol and chloroform/methanol/water (3:5:2) |
| [13] | milk of different species | Silica | hexane/diethyl-ether (8:2 and 1:1) | methanol and chloroform/methanol/water (3:5:2) |
| [31] | milk | Silica | chloroform/methanol (95:5) | methanol and chloroform/methanol/water (3:5:2) |
| Reference | Matrix | HPLC Column phase | HPLC mobile phase | ELSD: temperature/pressure or flow | PL identification | Molecules identified in samples |
|---|---|---|---|---|---|---|
| [91] | Egg powder, chicken meat, cheese, salami | Silica | Solvent A: chloroform/metanol/NH4OH (80:19.5:0.5) Solvent B: chloroform/methanol/water/NH4OH (60:34:5.5:0.5) | 60 °C/2 atm | authentic standards | PE, PC, PI, PG, SM, LPC |
| [22] | milk, cream, butter, fresh buttermilk | Silica | Solvent A: chloroform/metanol/NH4OH (80:19.5:0.5) Solvent B: chloroform/methanol/water/NH4OH (60:34:5.5:0.5) | 50 °C/2.2 bar | authentic standards | PE, PC, PI, PS, SM |
| [25] | milk, cream, butter, cheese, whey, yoghurt, fermented buttermilk | Silica | chloroform/methanol/buffer (1M formic acid, neutralized to pH 3 with triethylamine) (87.5:12:0.5) | 85 °C/1.4 L/min | authentic standards | PE, PC, PI, PS, SM, GluCer, LacCer |
| [28] | milk of different species, powdered buttermilk | Silica | Solvent A: chloroform/methanol/water (1M formic acid; triethylamine) (87.5:12:0.5). Solvent B: chloroform/methanol/water (1M formic acid; triethylamine) (28:60:12). Solvent C: isooctane/tetrahydrofurane (99:1). Solvent D: 2-Propanol | 60 °C/3.5 bar | authentic standards | PE, PC, PI, PS, SM, LacCer |
| [29] | milk, cheese, butter | Silica | Solvent A: dichloromethane Solvent B: methanol/buffer (7.2 mL acetic acid, 8.0 ml triethylamine and 118 mL HPLC water) (500:21) | 65 °C/2.1 L/min | authentic standards | PE, PC, PI, PS, SM, GluCer, LacCer |
| [30] | milk of different species | HILIC * | Solvent A: acetonitrile Solvent B: acetonitrile/water (2:1) | 50 °C/180 KPa | ESI/TOF | PE, PC, PI, PS, SM |
| [31] | milk | Silica | Solvent A:13% formic acid Solvent B: hexane Solvent C: 2-propanol | CAD detector (see text) | authentic standards | PE, PC, PI, PS, SM |
| Reference | Matrix | PC | PE | PI | PS | SM | DHSM | EPLAS | LPC | LPE | PA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [11] | cow milk | 26.8 | 25.8 | 14.0 | 1.5 | 26.8 | 4.6 | 0.5 | |||
| [23] | cow milk | 24.0 | 23.5 | 12.0 | 3.6 | 24.2 | |||||
| [13] | cow milk | 28.7 | 31.4 | 3.6 | 11.2 | 19.9 | 4.5 | ||||
| [34] | cow cream | 26.5 | 26.7 | 7.5 | 11.7 | 20.8 | 3.9 | 1.1 | 1.8 | ||
| [11] | ewe milk | 23.9 | 27.5 | 9.4 | 3.9 | 28.3 | 6.5 | 0.5 | |||
| [23] | buffalo milk | 21.6 | 21.8 | 17.5 | 5.9 | 22.1 | |||||
| [13] | camel milk | 19.3 | 30.0 | 4.3 | 9.2 | 24.6 | 6.4 | 0.9 | |||
| [13] | mare milk | 21.3 | 18.6 | 6.5 | 8.1 | 22.2 | 3.4 | 1.0 | 8.3 | 3.1 | |
| [13] | human milk | 24.5 | 18.3 | 3.8 | 8.1 | 29.7 | 11.4 | 2.5 | |||
| [34] | buttermilk (lipid fraction) | 27.0 | 25.7 | 5.8 | 9.7 | 20.4 | 4.6 | 0.7 | 1.0 | ||
| [34] | buttermilk (direct analysis) | 26.4 | 25.8 | 7.6 | 11.5 | 16.9 | 4.6 | 0.7 | 1.0 |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Contarini, G.; Povolo, M. Phospholipids in Milk Fat: Composition, Biological and Technological Significance, and Analytical Strategies. Int. J. Mol. Sci. 2013, 14, 2808-2831. https://doi.org/10.3390/ijms14022808
Contarini G, Povolo M. Phospholipids in Milk Fat: Composition, Biological and Technological Significance, and Analytical Strategies. International Journal of Molecular Sciences. 2013; 14(2):2808-2831. https://doi.org/10.3390/ijms14022808
Chicago/Turabian StyleContarini, Giovanna, and Milena Povolo. 2013. "Phospholipids in Milk Fat: Composition, Biological and Technological Significance, and Analytical Strategies" International Journal of Molecular Sciences 14, no. 2: 2808-2831. https://doi.org/10.3390/ijms14022808
APA StyleContarini, G., & Povolo, M. (2013). Phospholipids in Milk Fat: Composition, Biological and Technological Significance, and Analytical Strategies. International Journal of Molecular Sciences, 14(2), 2808-2831. https://doi.org/10.3390/ijms14022808