Evidence for the Involvement of RhoA Signaling in the Ethanol-Induced Increase in Intestinal Epithelial Barrier Permeability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cell Viability

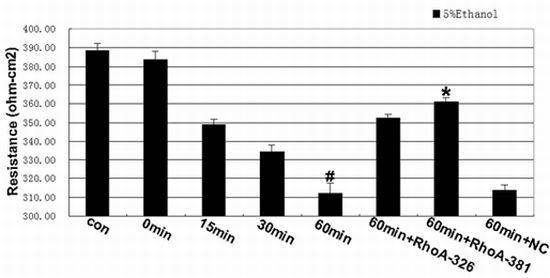
2.2. Effect of RhoA on Ethanol-Induced IEB Permeability


2.3. RhoA Signal Transduction Activation

2.4. Inhibition of RhoA Prevented Ethanol-Induced MLC Phosphorylation

2.5. RhoA Activity Is Required for Ethanol-Induced Expression and Relocation of Occludin and ZO-1


3. Experimental Section
3.1. Culture of Caco-2 Cells and Establishment of an In Vitro IEB Model
3.2. Viability Assay
3.3. Measurement of Transepithelial Electrical Resistance (TEER) and Fluorescent Yellow Flux Rate Measurement
3.4. RNA Interference
| Group | shRNA sequences |
|---|---|
| U6/GFP/Neo-RHOA-homo-326 | S5'-CACCGAAAGACATGCTTGCTCATAGTTCAAGAGACTATG AGCAAGCATGTCTTTCTTTTTTG-3' |
| A5'-GATCCAAAAAAGAAAGACATGCTTGCTCATAGTCTCTTGA ACTATGAGCAAGCATGTCTTTC-3' | |
| U6/GFP/Neo-RHOA-homo-381 | S5'-CACCGCCCACAGTGTTTGAGAACTATTCAAGAGATAGTTC TCAAACACTGTGGGCTTTTTTG-3' |
| A5'-GATCCAAAAAAGCCCACAGTGTTTGAGAACTATCTCTTGA ATAGTTCTCAAACACTGTGGGC-3' | |
| pGPU6/GFP/Neo-shNC | S5'-CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGAC ACGTTCGGAGAATTTTTTG-3' |
| A5'-GATCCAAAAAATTCTCCGAACGTGTCACGTAATCTCTTGA CGTGACACGTTCGGAGAAC-3' |
3.5. Treatment and Experimental Groups
3.6. Cell Fractionation and Western Blot Analysis
3.7. RhoA Activation Assay
3.8. Immunofluorescence
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Anderson, J.M.; van Itallie, C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995, 269, G467–G475. [Google Scholar]
- Atkinson, K.J.; Rao, R.K. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am. J. Physiol. 2001, 280, G1280–G1288. [Google Scholar]
- Ma, T.Y.; Nguyen, D.; Bui, V.; Nguyen, H.; Hoa, N. Ethanol modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. 1999, 276, G965–G974. [Google Scholar]
- Rao, R.K. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol. Clin. Exp. Res. 1998, 22, 1724–1730. [Google Scholar] [CrossRef]
- Elamin, E.; Jonkers, D.; Juuti-Uusitalo, K.; van Ijzendoorn, S.; Troost, F.; Duimel, H.; Broers, J.; Verheyen, F.; Dekker, J.; Masclee, A. Effects of ethanol and acetaldehyde on tight junction integrity: In vitro study in a three dimensional intestinal epithelial cell culture model. PLoS One 2012, 7, e35008. [Google Scholar]
- Greenberg, S.S.; Xie, J.; Wang, Y.; Malinski, T.; Summer, W.R.; McDonough, K. Escherichia coli-induced inhibition of endothelium-dependent relaxation and gene expression and release of nitric oxide is attenuated by chronic alcohol ingestion. Alcohol 1994, 11, 53–60. [Google Scholar] [CrossRef]
- Sisson, J.H. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. Am. J. Physiol. 1995, 268, L596–L600. [Google Scholar]
- Chow, J.Y.; Ma, L.; Cho, C.H. Effect of cigarette smoke on ethanol-induced gastric mucosal lesions: The role of nitric oxide and neutrophils. Eur. J. Pharmacol. 1998, 342, 253–260. [Google Scholar] [CrossRef]
- Banan, A.; Choudhary, S.; Zhang, Y.; Fields, J.Z.; Keshavarzian, A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: Evidence for oxidative and cytoskeletal mechanisms. J. Pharmacol. Exp. Ther. 1999, 291, 1075–1085. [Google Scholar]
- Banan, A.; Fields, J.Z.; Decker, H.; Zhang, Y.; Keshavarzian, A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J. Pharmacol. Exp. Ther. 2000, 294, 997–1008. [Google Scholar]
- Keshavarzian, A.; Farhadi, A.; Forsyth, C.B.; Rangan, J.; Jakate, S.; Shaikh, M.; Banan, A.; Fields, J.Z. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J. Hepatol. 2009, 50, 538–547. [Google Scholar]
- Terry, S.; Nie, M.; Matter, K.; Balda, M.S. Rho signaling and tight junction functions. Physiology (Bethesda) 2010, 25, 16–26. [Google Scholar] [CrossRef]
- Nusrat, A.; Giry, M.; Turner, J.R.; Colgan, S.P.; Parkos, C.A.; Carnes, D.; Lemichez, E.; Boquet, P.; Madara, J.L. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc. Natl. Acad. Sci. USA 1995, 92, 10629–10633. [Google Scholar]
- Jou, T.S.; Schneeberger, E.E.; Nelson, W.J. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J. Cell Biol. 1998, 142, 101–115. [Google Scholar] [CrossRef]
- Madara, J.L. Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 1998, 60, 143–159. [Google Scholar] [CrossRef]
- Hall, A. Rho GTPases and the actin cytoskeleton. Science 1998, 279, 509–514. [Google Scholar] [CrossRef]
- Rubenstein, N.M.; Chan, J.F.; Kim, J.Y.; Hansen, S.H.; Firestone, G.L. Rnd3/RhoE induces tight junction formation in mammary epithelial tumor cells. Exp. Cell Res. 2005, 305, 74–82. [Google Scholar] [CrossRef]
- Fujita, H.; Katoh, H.; Hasegawa, H.; Yasui, H.; Aoki, J.; Yamaguchi, Y.; Negishi, M. Molecular decipherment of Rho effector pathways regulating tight-junction permeability. Biochem. J. 2000, 346, 617–622. [Google Scholar] [CrossRef]
- Cui, W.; Li, L.X.; Sun, C.M.; Wen, Y.; Zhou, Y.; Dong, Y.L.; Liu, P. Tumor necrosis factor alpha increases epithelial barrier permeability by disrupting tight junctions in Caco-2 cells. Braz. J. Med. Biol. Res. 2010, 43, 330–337. [Google Scholar]
- Fish, S.M.; Proujansky, R.; Reenstra, W.W. Synergistic effects of interferon gamma and tumour necrosis factor alpha on T84 cell function. Gut 1999, 45, 191–198. [Google Scholar] [CrossRef]
- Hall, A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu. Rev. Cell Biol. 1994, 10, 31–54. [Google Scholar] [CrossRef]
- Narumiya, S. The small GTPase Rho: Cellular functions and signal transduction. J. Biochem. 1996, 120, 215–228. [Google Scholar] [CrossRef]
- Narumiya, S.; Ishizaki, T.; Watanabe, N. Rho effectors and reorganization of actin cytoskeleton. FEBS Lett. 1997, 410, 68–72. [Google Scholar] [CrossRef]
- Terry, S.J.; Zihni, C.; Elbediwy, A.; Vitiello, E.; Leefa Chong San, I.V.; Balda, M.S.; Matter, K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat. Cell Biol. 2011, 13, 159–166. [Google Scholar] [CrossRef]
- Niwa, R.; Nagata-Ohashi, K.; Takeichi, M.; Mizuno, K.; Uemura, T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell 2002, 108, 233–246. [Google Scholar] [CrossRef]
- Brown, M.; Adyshev, D.; Bindokas, V.; Moitra, J.; Garcia, J.G.; Dudek, S.M. Quantitative distribution and colocalization of non-muscle myosin light chain kinase isoforms and cortactin in human lung endothelium. Microvasc. Res. 2010, 80, 75–88. [Google Scholar] [CrossRef]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef]
- Ikebe, M.; Hartshorne, D.J. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J. Biol. Chem. 1985, 260, 10027–10031. [Google Scholar]
- Kimura, K.; Ito, M.; Amano, M.; Chihara, K.; Fukata, Y.; Nakafuku, M.; Yamamori, B.; Feng, J.; Nakano, T.; Okawa, K.; et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996, 273, 245–248. [Google Scholar]
- Moyer, R.A.; Wendt, M.K.; Johanesen, P.A.; Turner, J.R.; Dwinell, M.B. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab. Invest. 2007, 87, 807–817. [Google Scholar]
- Kubota, K.; Furuse, M.; Sasaki, H.; Sonoda, N.; Fujita, K.; Nagafuchi, A.; Tsukita, S. Ca2+-independent cell-adhesion activity of claudins, a family of integral membrane proteins localized at tight junctions. Curr. Biol. 1999, 9, 1035–1038. [Google Scholar]
- Bamburg, J.R. Proteins of the ADF/cofilin family: Essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 1999, 15, 185–230. [Google Scholar] [CrossRef]
- Nagumo, Y.; Han, J.; Bellila, A.; Isoda, H.; Tanaka, T. Cofilin mediates tight-junction opening by redistributing actin and tight-junction proteins. Biochem. Biophys. Res. Commun. 2008, 377, 921–925. [Google Scholar] [CrossRef]
- Olivera, D.; Knall, C.; Boggs, S.; Seagrave, J. Cytoskeletal modulation and tyrosine phosphorylation of tight junction proteins are associated with mainstream cigarette smoke-induced permeability of airway epithelium. Exp. Toxicol. Pathol. 2010, 62, 133–143. [Google Scholar] [CrossRef]
- Amano, M.; Ito, M.; Kimura, K.; Fukata, Y.; Chihara, K.; Nakano, T.; Matsuura, Y.; Kaibuchi, K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 1996, 271, 20246–20249. [Google Scholar]
- Collares-Buzato, C.B.; Jepson, M.A.; Simmons, N.L.; Hirst, B.H. Increased tyrosine phosphorylation causes redistribution of adherens junction and tight junction proteins and perturbs paracellular barrier function in MDCK epithelia. Eur. J. Cell Biol. 1998, 76, 85–92. [Google Scholar] [CrossRef]
- Keita, A.V.; Soderholm, J.D. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 2010, 22, 718–733. [Google Scholar] [CrossRef]
- Morita, K.; Sasaki, H.; Furuse, M.; Tsukita, S. Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 1999, 147, 185–194. [Google Scholar] [CrossRef]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef]
- Luabeya, M.K.; Dallasta, L.M.; Achim, C.L.; Pauza, C.D.; Hamilton, R.L. Blood-brain barrier disruption in simian immunodeficiency virus encephalitis. Neuropathol. Appl. Neurobiol. 2000, 26, 454–462. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S.; Davies, D.C.; Bell, B.A. Emerging molecular mechanisms of brain tumour oedema. Br. J. Neurosurg. 2001, 15, 101–108. [Google Scholar] [CrossRef]
- Hirase, T.; Kawashima, S.; Wong, E.Y.; Ueyama, T.; Rikitake, Y.; Tsukita, S.; Yokoyama, M.; Staddon, J.M. Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and -independent mechanisms. J. Biol. Chem. 2001, 276, 10423–10431. [Google Scholar]
- Andreeva, A.Y.; Krause, E.; Muller, E.C.; Blasig, I.E.; Utepbergenov, D.I. Protein kinase C regulates the phosphorylation and cellular localization of occludin. J. Biol. Chem. 2001, 276, 38480–38486. [Google Scholar]
- Seth, A.; Sheth, P.; Elias, B.C.; Rao, R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J. Biol. Chem. 2007, 282, 11487–11498. [Google Scholar]
- Sakakibara, A.; Furuse, M.; Saitou, M.; Ando-Akatsuka, Y.; Tsukita, S. Possible involvement of phosphorylation of occludin in tight junction formation. J. Cell Biol. 1997, 137, 1393–1401. [Google Scholar] [CrossRef]
- Nighot, P.K.; Moeser, A.J.; Ryan, K.A.; Ghashghaei, T.; Blikslager, A.T. ClC-2 is required for rapid restoration of epithelial tight junctions in ischemic-injured murine jejunum. Exp. Cell Res. 2009, 315, 110–118. [Google Scholar] [CrossRef]
- Tenenbaum, T.; Matalon, D.; Adam, R.; Seibt, A.; Wewer, C.; Schwerk, C.; Galla, H.J.; Schroten, H. Dexamethasone prevents alteration of tight junction-associated proteins and barrier function in porcine choroid plexus epithelial cells after infection with Streptococcus suis in vitro. Brain Res. 2008, 1229, 1–17. [Google Scholar] [CrossRef]
- Yasuda, Y.; Saito, M.; Yamamura, T.; Yaguchi, T.; Nishizaki, T. Extracellular adenosine induces apoptosis in Caco-2 human colonic cancer cells by activating caspase-9/-3 via A(2a) adenosine receptors. J. Gastroenterol. 2009, 44, 56–65. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tong, J.; Wang, Y.; Chang, B.; Zhang, D.; Wang, B. Evidence for the Involvement of RhoA Signaling in the Ethanol-Induced Increase in Intestinal Epithelial Barrier Permeability. Int. J. Mol. Sci. 2013, 14, 3946-3960. https://doi.org/10.3390/ijms14023946
Tong J, Wang Y, Chang B, Zhang D, Wang B. Evidence for the Involvement of RhoA Signaling in the Ethanol-Induced Increase in Intestinal Epithelial Barrier Permeability. International Journal of Molecular Sciences. 2013; 14(2):3946-3960. https://doi.org/10.3390/ijms14023946
Chicago/Turabian StyleTong, Jing, Ying Wang, Bing Chang, Dai Zhang, and Bingyuan Wang. 2013. "Evidence for the Involvement of RhoA Signaling in the Ethanol-Induced Increase in Intestinal Epithelial Barrier Permeability" International Journal of Molecular Sciences 14, no. 2: 3946-3960. https://doi.org/10.3390/ijms14023946
APA StyleTong, J., Wang, Y., Chang, B., Zhang, D., & Wang, B. (2013). Evidence for the Involvement of RhoA Signaling in the Ethanol-Induced Increase in Intestinal Epithelial Barrier Permeability. International Journal of Molecular Sciences, 14(2), 3946-3960. https://doi.org/10.3390/ijms14023946