Anti-Inflammatory Components from the Root of Solanum erianthum
Abstract
:1. Introduction
2. Results and Discussion
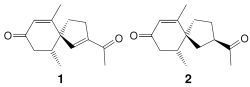
2.1. Isolation and Structure Elucidation
2.2. Anti-Inflammatory Activities
2.3. Cytotoxicity Assay
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Solanerianone A (1)
3.5. Solanerianone B (2)
3.6. Anti-Inflammatory Activity Assay
3.6.1. Cell Culture
3.6.2. Evaluation of NO Product by Nitrite Measurement
3.6.3. Cell Viability Assay
3.7. Cytotoxicity Assay
3.7.1. Cell Culture
3.7.2. Mitochondrial Reductase Activity
3.8. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-14-12581-s001.pdfAcknowledgments
Conflict of Interest
References
- William, G.D.; Peng, C.I. Solanaceae. In Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1998; Volume 4, pp. 566–567. [Google Scholar]
- Ajasa, A.M.O.; Bello, M.O.; Ibrahim, A.O.; Ogunwande, I.A.; Olawore, N.O. Heavy trace metals and macronutrients status in herbal plants of Nigeria. Food Chem 2004, 85, 67–71. [Google Scholar]
- Kao, M.T. Handbook of Medicinal Plants in Taiwan, 5th ed.; SMC Publishing Inc.: Taipei, Taiwan, 1990; p. 372. [Google Scholar]
- Chang, Y.S.; Chen, I.S.; Hsieh, W.C.; Ou, J.C. The Catalogue of Medicinal Plant Resources in Taiwan, 1st ed.; Committee on Chinese Medicine and Pharmacy, Department of Health, Executive Yuan: Taipei, Taiwan, 2003; p. 405. [Google Scholar]
- Tuan, N.H.; Than, N.V.; Thuan, N.D.; Minh, C.V.; Kiem, P.V. A new C-methylflavonol from the leaves of Solanum erianthum D. Don. Adv. Nat. Sci 2008, 9, 163–169. [Google Scholar]
- Nguyen, H.T.; Nguyen, V.T.; Nguyen, D.T.; Chau, V.M.; Phan, V.K. Two steroidal alkaloids, solansonine and solamargine, from Solanum erianthum D. Don. Tap Chi Duoc Hoc 2008, 48, 31–36. [Google Scholar]
- Ali, M.S.; Shahnaz Tabassum, S.; Ogunwande, I.A.; Pervez, M.K.; Ibrahim, O.A. Naturally occurring antifungal aromatic esters and amides. J. Chem. Soc. Pak 2010, 32, 565–570. [Google Scholar]
- Chou, S.C.; Huang, T.J.; Lin, E.H.; Huang, C.H.; Chou, C.H. Antihepatitis B virus constituents of Solanum erianthum. Nat. Prod. Commun 2012, 7, 153–156. [Google Scholar]
- Moreira, E.; Cecy, C.; Nakashima, T.; Cavazzani, J.R.; Miguel, O.G.; Krambeck, R. Solasodine in Solanum erianthum D. Don. Trib. Farm 1980, 48, 61–84. [Google Scholar]
- Mahadev, R.; Ramakrishnaiah, H.; Krishna, V.; Kumar, N.N. Chemical composition of the essential oil from the fruits of Solanum erianthum D. Don. J. Essent. Oil Bear. Pl 2012, 15, 387–391. [Google Scholar]
- Essien, E.E.; Ogunwande, I.A.; Setzer, W.N.; Ekundayo, O. Chemical composition, antimicrobial, and cytotoxicity studies on S. erianthum and S. macranthum essential oils. Pharm. Biol 2012, 50, 474–480. [Google Scholar]
- Coxon, D.T.; Price, K.R.; Howard, B.; Osman, S.F.; Kalan, E.B.; Zacharius, R.M. Two new vetispirane derivatives: stress metabolites from potato (Solanum tuberosum) tubers. Tetrahedron Lett 1974, 34, 2921–2924. [Google Scholar]
- Hwu, J.R.; Wetzel, J.M. Silicon-promoted ring contractions in the formation of carbocyclic spiro compounds. Total synthesis of (−)-solavetivone. J. Org. Chem 1992, 57, 922–928. [Google Scholar]
- Engstrom, K. Sesquiterpenoid spiro compounds from potato tubers infected with Phoma foveata and Fusarium spp. Phytochemistry 1998, 47, 985–990. [Google Scholar]
- Blay, G.; Cardona, L.; Collado, A.M.; García, B.; Morcillo, V.; Pedro, J.R. Synthesis of spirovetivane sesquiterpenes from santonin. Synthesis of (+)-anhydro-β-rotunol and all diastereomers of 6,11-spirovetivadiene. J. Org. Chem 2004, 69, 7294–7302. [Google Scholar]
- Syu, W.J.; Don, M.J.; Lee, G.H.; Sun, C.M. Cytotoxic and novel compounds from Solanum indicum. J. Nat. Prod 2001, 64, 1232–1233. [Google Scholar]
- Guo, F.; Li, Y. New sesquiterpenoids from Lycianthes marlipoensis. Helv. Chim. Acta 2005, 88, 2364–2369. [Google Scholar]
- Yoshihara, T.; Yamaguchi, K.; Takamatsu, S.; Sakamura, S. A new lignan amide, grossamide, from bell pepper (Capsicum annuum var. grossum). Agric. Biol. Chem 1981, 45, 2593–2598. [Google Scholar]
- Kuo, Y.H.; Chu, P.H. Studies on the constituents from the bark of Bauhinia purpurea. J. Chin. Chem. Soc 2002, 49, 269–274. [Google Scholar]
- Gómez-Cordovés, C.; Bartolomé, B.; Jimeno, M.L. Identification of 2,3-dihydroxy-1-guaiacylpropan-1-one in brandies. J. Agric. Food Chem 1997, 45, 873–876. [Google Scholar]
- Kojima, H.; Sato, N.; Hatano, A.; Ogura, H. Sterol glucosides from Prunella vulgaris. Phytochemistry 1990, 29, 2351–2355. [Google Scholar]
- Wu, Y.C.; Chang, G.Y.; Ko, F.N.; Teng, C.M. Bioactive constitutents from the stems of Annona montana. Planta Med 1995, 61, 146–149. [Google Scholar]
- Wang, G.J.; Chen, Y.M.; Wang, T.M.; Lee, C.K.; Chen, K.J.; Lee, T.H. Flavonoids with iNOS inhibitory activity from Pogonatherum crinitum. J. Ethnopharmacol 2008, 118, 71–78. [Google Scholar]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Wishnik, I.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem 1982, 126, 131–138. [Google Scholar]
- Kwack, K.; Lynch, R.G. A new non-radioactive method for IL-2 bioassay. Mol. Cells 2000, 10, 575–578. [Google Scholar]
- Kuroyanagi, M.; Arakawa, T.; Mikami, Y.; Yoshida, K.; Kawahar, N.; Hayashi, T.; Ishimaru, H. Phytoalexins from hairy roots of Hyoscyamus albus treated with methyl jasmonate. J. Nat. Prod 1998, 61, 1516–1519. [Google Scholar]
- Morita, M.; Nakanishi, H.; Morita, H.; Mihashi, S.; Itokawa, H. Structures and spasmolytic activities of derivatives from sesquiterpenes of Alpinia speciosa and Alpinia japonica. Chem. Pharm. Bull 1996, 44, 1603–1606. [Google Scholar]
- Ueda, J.; Imamura, L.; Tezuka, Y.; Tran, Q.L.; Tsuda, M.; Kadota, S. New sesquiterpene from Vietnamese agarwood and its induction effect on brain-derived neurotrophic factor mRNA expression in vitro. Bioorg. Med. Chem 2006, 14, 3571–3574. [Google Scholar]
- Tamariz, J.; Schwager, L.; Stibbard, J.H.A.; Vogel, P. Control of diels-alder addition regioselectivity by remote substituents. Synthesis of anthracycline precursors. Tetrahedron Lett 1983, 24, 1497–1500. [Google Scholar]




| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 6.34 (t, J = 1.8, 1H) | 145.7 | 1.96 (m, 1H) | 37.5 |
| 2.13 (m, 1H) | ||||
| 2 | - | 147.4 | 3.05 (m, 1H) | 51.4 |
| 3 | 2.60 (m, 1H) | 31.2 | 1.91 (m, 1H) | 29.4 |
| 2.72 (m, 1H) | 2.01 (m, 1H) | |||
| 4 | 1.79 (ddd, J = 14.4, 9.6, 7.2, 1H) | 27.2 | 1.75 (m, 1H) | 33.2 |
| 2.22 (ddd, J = 14.4, 9.6, 4.8, 1H) | 1.89 (m, 1H) | |||
| 5 | - | 59.4 | - | 50.8 |
| 6 | - | 165.5 | - | 165.1 |
| 7 | 5.85 (br s, 1H) | 126.1 | 5.80 (br s, 1H) | 126.4 |
| 8 | - | 198.6 | - | 198.6 |
| 9 | 2.28 (br d, J = 13.2, 1H) | 41.9 | 2.23 (dd, J = 16.8, 4.8, 1H) | 42.6 |
| 2.41 (br d, J = 13.2, 1H) | 2.64 (dd, J = 16.8, 4.8, 1H) | |||
| 10 | 2.31 (qt, J = 6.0, 1.8, 1H) | 37.2 | 2.11 (m, 1H) | 37.7 |
| 11 | - | 196.4 | - | 209.6 |
| 12 | 2.38 (s, 3H) | 27.0 | 2.20 (s, 3H) | 29.4 |
| 13 | 1.87 (d, J = 1.8, 3H) | 21.4 | 1.93 (d, J = 1.2, 3H) | 21.0 |
| 14 | 0.92 (d, J = 6.0, 3H) | 15.9 | 0.99 (d, J = 7.2, 3H) | 15.9 |
| Compound | Emax (%) | IC50 (μM) |
|---|---|---|
| (−)-Solavetivone (3) | 98.23 ± 0.08 | 65.54 ± 0.18 |
| (+)-Anhydro-β-rotunol (4) | 8.77 ± 1.24 | >100 |
| Solafuranone (5) | 0 ± 0 | >100 |
| Lycifuranone A (6) | 0 ± 0 | >100 |
| N-trans-Feruloyltyramine (7) | 33.33 ± 1.69 | >100 |
| Palmitic acid (8) | 13.22 ± 1.11 | >100 |
| Positive control aminoguanidine (a selective iNOS inhibitor) | 80.97 ± 0.63 | 22.28 ± 0.47 |
| Nω-nitro-l-arginine (a nonselective iNOS inhibitor) | 42.19 ± 0.94 | 147.33 ± 7.61 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Y.-C.; Lee, H.-Z.; Chen, H.-C.; Wen, C.-L.; Kuo, Y.-H.; Wang, G.-J. Anti-Inflammatory Components from the Root of Solanum erianthum. Int. J. Mol. Sci. 2013, 14, 12581-12592. https://doi.org/10.3390/ijms140612581
Chen Y-C, Lee H-Z, Chen H-C, Wen C-L, Kuo Y-H, Wang G-J. Anti-Inflammatory Components from the Root of Solanum erianthum. International Journal of Molecular Sciences. 2013; 14(6):12581-12592. https://doi.org/10.3390/ijms140612581
Chicago/Turabian StyleChen, Yu-Chang, Hong-Zin Lee, Hsin-Chun Chen, Chi-Luan Wen, Yueh-Hsiung Kuo, and Guei-Jane Wang. 2013. "Anti-Inflammatory Components from the Root of Solanum erianthum" International Journal of Molecular Sciences 14, no. 6: 12581-12592. https://doi.org/10.3390/ijms140612581
APA StyleChen, Y. -C., Lee, H. -Z., Chen, H. -C., Wen, C. -L., Kuo, Y. -H., & Wang, G. -J. (2013). Anti-Inflammatory Components from the Root of Solanum erianthum. International Journal of Molecular Sciences, 14(6), 12581-12592. https://doi.org/10.3390/ijms140612581