Intravitreal Injection of Ranibizumab and CTGF shRNA Improves Retinal Gene Expression and Microvessel Ultrastructure in a Rodent Model of Diabetes
Abstract
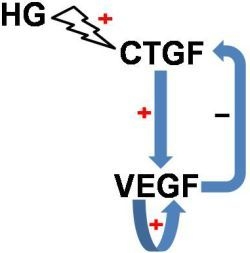
:1. Introduction
2. Results
2.1. Blood Glucose Level of Streptozocin-Induced Diabetic Rats
2.2. Ultrastructural Changes in Retinal Microvessel of Diabetic Rats
2.3. Expression of VEGF and CTGF Genes in Retina of Diabetic Rats
2.4. Efficacy of Anti-CTGF shRNA
2.5. Distribution of the Vector Encoding Anti-CTGF shRNA in Diabetic Retina
2.6. Effects of Dual-Target Intervention on VEGF and CTGF Expression in Retina of Diabetic Rats
2.7. Effects of Dual-Target Intervention on Ultrastructural Changes in Retina of Diabetic Rats
3. Discussion
4. Experimental Section
4.1. Animals
4.2. Induction of Diabetes
4.3. Design of shRNA against Rat CTGF
4.4. Efficiency of Anti-CTGF shRNA in Cells Overexpressing Rat CTGF
4.5. Intravitreal Injection of CTGF shRNA and Ranibizumab
4.6. RNA Extraction and Reverse Transcription
4.7. Real-Time PCR Analyses for CTGF and VEGF Expression
4.8. Transmission Electron Microscopy
4.9. Retinal Cryosections, Immunofluorescence and Fluorescence Microscopy
4.10. Statistics
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med 2012, 366, 1227–1239. [Google Scholar]
- Adamis, A.P.; Berman, A.J. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin. Immunopathol 2008, 30, 65–84. [Google Scholar]
- Bringmann, A.; Reichenbach, A.; Wiedemann, P. Pathomechanisms of cystoid macular edema. Ophthalmic Res 2004, 36, 241–249. [Google Scholar]
- Madsen-Bouterse, S.A.; Kowluru, R.A. Oxidative stress and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Rev. Endocr. Metab. Disord 2008, 9, 315–327. [Google Scholar]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res 2010, 106, 1319–1331. [Google Scholar]
- Drel, V.R.; Xu, W.; Zhang, J.; Kador, P.F.; Ali, T.K.; Shin, J.; Julius, U.; Slusher, B.; El-Remessy, A.B.; Obrosova, I.G. Poly(ADP-ribose)polymerase inhibition counteracts cataract formation and early retinal changes in streptozotocin-diabetic rats. Invest. Ophthalmol. Vis. Sci 2009, 50, 1778–1790. [Google Scholar]
- Lorenzi, M. The polyol pathway as a mechanism for diabetic retinopathy: Attractive, elusive, and resilient. Exp. Diabetes Res 2007, 2007, 61038. [Google Scholar]
- Querques, G.; Delle, N.N. Proinflammatory cytokines and angiogenic and antiangiogenic factors in vitreous of patients with proliferative diabetic retinopathy and Eales’ disease (ED). Retina 2009, 29, 121–123. [Google Scholar]
- Kita, T.; Hata, Y.; Kano, K.; Miura, M.; Nakao, S.; Noda, Y.; Shimokawa, H.; Ishibashi, T. Transforming growth factor-beta2 and connective tissue growth factor in proliferative vitreoretinal diseases: Possible involvement of hyalocytes and therapeutic potential of Rho kinase inhibitor. Diabetes 2007, 56, 231–238. [Google Scholar]
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. (Lond.) 2005, 109, 227–241. [Google Scholar]
- Altintas, A.G.; Arifoglu, H.B.; Tutar, E.; Koklu, G.; Ozcan, P.Y. Effect on anterior chamber bevacizumab injection combined with seton implantation in treatment of rubeosis iridis in neovascular glaucoma. Cutan. Ocul. Toxicol 2012, 31, 124–127. [Google Scholar]
- Sugimoto, Y.; Mochizuki, H.; Okumichi, H.; Takumida, M.; Takamatsu, M.; Kawamata, S.; Kiuchi, Y. Effect of intravitreal bevacizumab on iris vessels in neovascular glaucoma patients. Graefes Arch. Clin. Exp. Ophthalmol 2010, 248, 1601–1609. [Google Scholar]
- Yoon, J.U.; Kim, Y.M.; Lee, S.J.; Byun, Y.J.; Koh, H.J. Prognostic factors for visual outcome after intravitreal anti-VEGF injection for naive myopic choroidal neovascularization. Retina 2012, 32, 949–955. [Google Scholar]
- Pi, L.; Xia, H.; Liu, J.; Shenoy, A.K.; Hauswirth, W.W.; Scott, E.W. Role of connective tissue growth factor in the retinal vasculature during development and ischemia. Invest. Ophthalmol. Vis. Sci 2011, 52, 8701–8710. [Google Scholar]
- Grote, K.; Salguero, G.; Ballmaier, M.; Dangers, M.; Drexler, H.; Schieffer, B. The angiogenic factor CCN1 promotes adhesion and migration of circulating CD34+ progenitor cells: Potential role in angiogenesis and endothelial regeneration. Blood 2007, 110, 877–885. [Google Scholar]
- Shimo, T.; Nakanishi, T.; Nishida, T.; Asano, M.; Kanyama, M.; Kuboki, T.; Tamatani, T.; Tezuka, K.; Takemura, M.; Matsumura, T.; et al. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J. Biochem 1999, 126, 137–145. [Google Scholar]
- Braig, S.; Wallner, S.; Junglas, B.; Fuchshofer, R.; Bosserhoff, A.K. CTGF is overexpressed in malignant melanoma and promotes cell invasion and migration. Br. J. Cancer 2011, 105, 231–238. [Google Scholar]
- Lasky, J.A.; Ortiz, L.A.; Tonthat, B.; Hoyle, G.W.; Corti, M.; Athas, G.; Lungarella, G.; Brody, A.; Friedman, M. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am. J. Physiol 1998, 275, L365–L371. [Google Scholar]
- van Setten, G.B.; Blalock, T.D.; Grotendorst, G.; Schultz, G.S. Detection of connective tissue growth factor in human aqueous humor. Ophthalmic Res 2002, 34, 306–308. [Google Scholar]
- van Setten, G.B.; Blalock, T.D.; Grotendorst, G.; Schultz, G.S. Detection of connective tissue growth factor (CTGF) in human tear fluid: Preliminary results. Acta Ophthalmol. Scand 2003, 81, 51–53. [Google Scholar]
- Abu El-Asrar, A.M.; Imtiaz, N.M.; Kangave, D.; Siddiquei, M.M.; Geboes, K. Osteopontin and other regulators of angiogenesis and fibrogenesis in the vitreous from patients with proliferative vitreoretinal disorders. Mediators Inflamm 2012, 2012, 493043. [Google Scholar]
- Tikellis, C.; Cooper, M.E.; Twigg, S.M.; Burns, W.C.; Tolcos, M. Connective tissue growth factor is up-regulated in the diabetic retina: Amelioration by angiotensin-converting enzyme inhibition. Endocrinology 2004, 145, 860–866. [Google Scholar]
- Van Geest, R.J.; Lesnik-Oberstein, S.Y.; Tan, H.S.; Mura, M.; Goldschmeding, R.; van Noorden, C.J.; Klaassen, I.; Schlingemann, R.O. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br. J. Ophthalmol 2012, 96, 587–590. [Google Scholar]
- Kuiper, E.J.; van Nieuwenhoven, F.A.; de Smet, M.D.; van Meurs, J.C.; Tanck, M.W.; Oliver, N.; Klaassen, I.; van Noorden, C.J.; Goldschmeding, R.; Schlingemann, R.O. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One 2008, 3, e2675. [Google Scholar]
- Arevalo, J.F.; Maia, M.; Flynn, H.W., Jr.; Saravia, M.; Avery, R.L.; Wu, L.; Eid Farah, M.; Pieramici, D.J.; Berrocal, M.H.; Sanchez, J.G. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br. J. Ophthalmol 2008, 92, 213–216. [Google Scholar]
- El-Sabagh, H.A.; Abdelghaffar, W.; Labib, A.M.; Mateo, C.; Hashem, T.M.; Al-Tamimi, D.M.; Selim, A.A. Preoperative intravitreal bevacizumab use as an adjuvant to diabetic vitrectomy: Histopathologic findings and clinical implications. Ophthalmology 2011, 118, 636–641. [Google Scholar]
- Wu, L.; Martinez-Castellanos, M.A.; Quiroz-Mercado, H.; Arevalo, J.F.; Berrocal, M.H.; Farah, M.E.; Maia, M.; Roca, J.A.; Rodriguez, F.J. Twelve-month safety of intravitreal injections of bevacizumab (Avastin): Results of the Pan-American Collaborative Retina Study Group (PACORES). Graefes Arch. Clin. Exp. Ophthalmol 2008, 246, 81–87. [Google Scholar]
- Hu, B.J.; Zeng, Q.; Liu, X.L.; Li, X.R.; Song, W.J. Influence of intravitreal injection of avastin on the expression of cytokines in fibrovascular membrane in proliferative diabetic retinopathy. Chin. J. Exp. Ophthalmol 2013, 31, 55–59. [Google Scholar]
- Rodrigues, E.B.; Farah, M.E.; Maia, M.; Penha, F.M.; Regatieri, C.; Melo, G.B.; Pinheiro, M.M.; Zanetti, C.R. Therapeutic monoclonal antibodies in ophthalmology. Prog. Retin. Eye Res 2009, 28, 117–144. [Google Scholar]
- Winkler, J.L.; Kedees, M.H.; Guz, Y.; Teitelman, G. Inhibition of connective tissue growth factor by small interfering ribonucleic acid prevents increase in extracellular matrix molecules in a rodent model of diabetic retinopathy. Mol. Vis 2012, 18, 874–886. [Google Scholar]
- Arevalo, J.F.; Sanchez, J.G.; Wu, L.; Maia, M.; Alezzandrini, A.A.; Brito, M.; Bonafonte, S.; Lujan, S.; Diaz-Llopis, M.; Restrepo, N.; et al. Primary intravitreal bevacizumab for diffuse diabetic macular edema: The Pan-American Collaborative Retina Study Group at 24 months. Ophthalmology 2009, 116, 1488–1497. [Google Scholar]
- Elman, M.J.; Qin, H.; Aiello, L.P.; Beck, R.W.; Bressler, N.M.; Ferris, F.L., III; Glassman, A.R.; Maturi, R.K.; Melia, M. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: Three-year randomized trial results. Ophthalmology 2012, 119, 2312–2318. [Google Scholar]
- Nguyen, Q.D.; Shah, S.M.; Khwaja, A.A.; Channa, R.; Hatef, E.; Do, D.V.; Boyer, D.; Heier, J.S.; Abraham, P.; Thach, A.B.; et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2010, 117, 2146–2151. [Google Scholar]
- Hwang, J.C.; Del Priore, L.V.; Freund, K.B.; Chang, S.; Iranmanesh, R. Development of subretinal fibrosis after anti-VEGF treatment in neovascular age-related macular degeneration. Ophthalmic Surg. Lasers Imaging 2011, 42, 6–11. [Google Scholar]
- Xu, H.; Czerwinski, P.; Hortmann, M.; Sohn, H.Y.; Förstermann, U.; Li, H. Protein kinase C alpha promotes angiogenic activity of human endothelial cells via induction of vascular endothelial growth factor. Cardiovasc. Res 2008, 78, 349–355. [Google Scholar]
- Judge, A.D.; Sood, V.; Shaw, J.R.; Fang, D.; McClintock, K.; MacLachlan, I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol 2005, 23, 457–462. [Google Scholar]
- Kuiper, E.J.; Hughes, J.M.; van Geest, R.J.; Vogels, I.M.; Goldschmeding, R.; van Noorden, C.J.; Schlingemann, R.O.; Klaassen, I. Effect of VEGF-A on expression of profibrotic growth factor and extracellular matrix genes in the retina. Invest. Ophthalmol. Vis. Sci 2007, 48, 4267–4276. [Google Scholar]
- Yang, H.; Huang, Y.; Chen, X.; Liu, J.; Lu, Y.; Bu, L.; Xia, L.; Xiao, W.; Chen, M.; Nie, Q.; et al. The role of CTGF in the diabetic rat retina and its relationship with VEGF and TGF-beta(2), elucidated by treatment with CTGFsiRNA. Acta. Ophthalmol 2010, 88, 652–659. [Google Scholar]
- Yang, H.W.; Chen, X.L.; Liu, Z.L.; Liu, J.; Bu, L.M. CTGF siRNA ameliorates retinal cells apoptosis in streptozotocin-induced diabetic rats. Int. J. Ophthalmol 2010, 3, 120–124. [Google Scholar]
- Lai, A.K.; Lo, A.C. Animal models of diabetic retinopathy: Summary and comparison. J. Diabetes Res 2013, 2013, 106594. [Google Scholar]
- Penn, J.S.; Tolman, B.L.; Henry, M.M. Oxygen-induced retinopathy in the rat: Relationship of retinal nonperfusion to subsequent neovascularization. Invest. Ophthalmol. Vis. Sci 1994, 35, 3429–3435. [Google Scholar]
- Shinohara, M.; Masuyama, T.; Shoda, T.; Takahashi, T.; Katsuda, Y.; Komeda, K.; Kuroki, M.; Kakehashi, A.; Kanazawa, Y. A new spontaneously diabetic non-obese Torii rat strain with severe ocular complications. Int. J. Exp. Diabetes Res 2000, 1, 89–100. [Google Scholar]
- Turchinovich, A.; Zoidl, G.; Dermietzel, R. Non-viral siRNA delivery into the mouse retina in vivo. BMC Ophthalmol. 2010, 10, 25. [Google Scholar]
- Banerjee, P.; Biswas, A.; Biswas, T. Porin-incorporated liposome induces Toll-like receptors 2- and 6-dependent maturation and type 1 response of dendritic cell. Int. Immunol 2008, 20, 1551–1563. [Google Scholar]
- Awasthi, S.; Cox, R.A. Transfection of murine dendritic cell line (JAWS II) by a nonviral transfection reagent. Biotechniques 2003, 35. [Google Scholar]
- Zhang, Y.; Wu, X.; He, Y.; Kastin, A.J.; Hsuchou, H.; Rosenblum, C.I.; Pan, W. Melanocortin potentiates leptin-induced STAT3 signaling via MAPK pathway. J. Neurochem 2009, 110, 390–399. [Google Scholar]
- Zhang, Y.; Kerman, I.A.; Laque, A.; Nguyen, P.; Faouzi, M.; Louis, G.W.; Jones, J.C.; Rhodes, C.; Münzberg, H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J. Neurosci 2011, 31, 1873–1884. [Google Scholar]
- Laque, A.; Zhang, Y.; Gettys, S.; Nguyen, T.A.; Bui, K.; Morrison, C.D.; Münzberg, H. Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. Am. J. Physiol. Endocrinol. Metab 2013, 304, E999–E1011. [Google Scholar]









© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, B.; Zhang, Y.; Zeng, Q.; Han, Q.; Zhang, L.; Liu, M.; Li, X. Intravitreal Injection of Ranibizumab and CTGF shRNA Improves Retinal Gene Expression and Microvessel Ultrastructure in a Rodent Model of Diabetes. Int. J. Mol. Sci. 2014, 15, 1606-1624. https://doi.org/10.3390/ijms15011606
Hu B, Zhang Y, Zeng Q, Han Q, Zhang L, Liu M, Li X. Intravitreal Injection of Ranibizumab and CTGF shRNA Improves Retinal Gene Expression and Microvessel Ultrastructure in a Rodent Model of Diabetes. International Journal of Molecular Sciences. 2014; 15(1):1606-1624. https://doi.org/10.3390/ijms15011606
Chicago/Turabian StyleHu, Bojie, Yan Zhang, Qing Zeng, Qian Han, Lijuan Zhang, Mian Liu, and Xiaorong Li. 2014. "Intravitreal Injection of Ranibizumab and CTGF shRNA Improves Retinal Gene Expression and Microvessel Ultrastructure in a Rodent Model of Diabetes" International Journal of Molecular Sciences 15, no. 1: 1606-1624. https://doi.org/10.3390/ijms15011606
APA StyleHu, B., Zhang, Y., Zeng, Q., Han, Q., Zhang, L., Liu, M., & Li, X. (2014). Intravitreal Injection of Ranibizumab and CTGF shRNA Improves Retinal Gene Expression and Microvessel Ultrastructure in a Rodent Model of Diabetes. International Journal of Molecular Sciences, 15(1), 1606-1624. https://doi.org/10.3390/ijms15011606