Fucoidan, a Sulfated Polysaccharide, Inhibits Osteoclast Differentiation and Function by Modulating RANKL Signaling
Abstract
:1. Introduction
2. Results
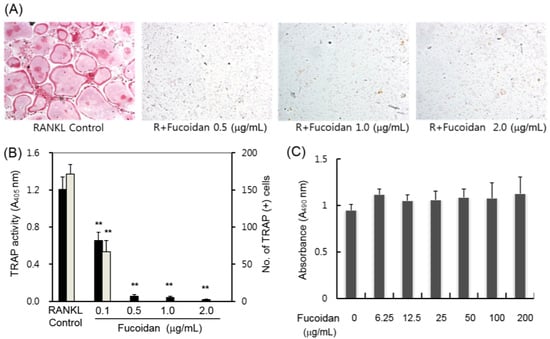
2.1. Inhibitory Effects of Fucoidan on Osteoclast Differentiation
2.2. Fucoidan Targets Early Stage Osteoclastogenesis


2.3. Fucoidan Down-Regulates RANKL-Induced Osteoclastogenesis-Related Genes

2.4. Fucoidan Inhibits Bone Resorption in Vitro

2.5. Fucoidan Down-Regulates RANKL-Induced C-Fos and NFATc1 Expression in BMMs

2.6. Fucoidan Inhibits the RANKL Induced Phosphorylation of MAPKs in BMMs
2.7. Fucoidan Inhibited RANKL Induced Nuclear Transport of NF-κB

3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Osteoclast Differentiation
4.3. Proliferation Assays
4.4. RT-PCR Analysis
4.5. Resorption Pit Assay
4.6. Western Blotting
4.7. Translocation of NF-κB
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wong, B.R.; Rho, J.; Arron, J.; Robinson, E.; Orlinick, J.; Chao, M.; Kalachikov, S.; Cayani, E.; Bartlett, F.S., III; Frankel, W.N.; et al. Trance is a novel ligand of the tumor necrosis factor receptor family that activates c-jun n-terminal kinase in T cells. J. Biol. Chem. 1997, 272, 25190–25194. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to trance/rankl. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Pharmacotherapy of osteoporosis in postmenopausal women: Focus on safety. Expert Opin. Drug Saf. 2002, 1, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.T. Postmenopausal hormone replacement therapy: Endometrial and breast effects. Adv. Anat. Pathol. 2007, 14, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Mourao, P.A. Use of sulfated fucans as anticoagulant and antithrombotic agents: Future perspectives. Curr. Pharm. Des. 2004, 10, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive effects of a fucoidan from brown alga undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The role of nk cells in antitumor activity of dietary fucoidan from undaria pinnatifida sporophylls (mekabu). Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-S.; Jung, W.-K.; Kim, J.-A.; Choi, I.L.W.; Kim, S.-K. Beneficial effects of fucoidan on osteoblastic mg-63 cell differentiation. Food Chem. 2009, 116, 990–994. [Google Scholar] [CrossRef]
- Changotade, S.I.; Korb, G.; Bassil, J.; Barroukh, B.; Willig, C.; Colliec-Jouault, S.; Durand, P.; Godeau, G.; Senni, K. Potential effects of a low-molecular-weight fucoidan extracted from brown algae on bone biomaterial osteoconductive properties. J. Biomed. Mater. Res. A 2008, 87, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Chang, U.J.; Lee, J.S. Inhibitory effects of fucoidan in 3t3-l1 adipocyte differentiation. Mar. Biotechnol. 2009, 11, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, O.H.; Lee, B.Y. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010, 86, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Mulloy, B.; Imai, K.; Tominaga, A.; Kaneko, T.; Asari, A.; Suzuki, K.; Masuda, H.; Kyogashima, M.; Ishii, T. Isolation and partial characterization of fucan sulfates from the body wall of sea cucumber stichopus japonicus and their ability to inhibit osteoclastogenesis. Carbohydr. Res. 2004, 339, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Franzoso, G.; Carlson, L.; Xing, L.; Poljak, L.; Shores, E.W.; Brown, K.D.; Leonardi, A.; Tran, T.; Boyce, B.F.; Siebenlist, U. Requirement for nf-kappab in osteoclast and b-cell development. Genes Dev. 1997, 11, 3482–3496. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor nfatc1 (nfat2) integrate rankl signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Ovitt, C.; Grigoriadis, A.E.; Mohle-Steinlein, U.; Ruther, U.; Wagner, E.F. Bone and haematopoietic defects in mice lacking c-fos. Nature 1992, 360, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Ranking c-jun in osteoclast development. J. Clin. Investig. 2004, 114, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.S.; Spiegelman, B.M.; Papaioannou, V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell 1992, 71, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Matsuo, K.; Suzuki, H.; Suzuki, T.; Sato, K.; Yokochi, T.; Oda, H.; Nakamura, K.; Ida, N.; et al. Rankl maintains bone homeostasis through c-fos-dependent induction of interferon-beta. Nature 2002, 416, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Galson, D.L.; Zhao, C.; Peng, L.; Laplace, C.; Wang, K.Z.; Bachler, M.A.; Amano, H.; Aburatani, H.; Ishikawa, H.; et al. Nuclear factor of activated t-cells (nfat) rescues osteoclastogenesis in precursors lacking c-fos. J. Biol. Chem. 2004, 279, 26475–26480. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Schinke, T.; Karsenty, G. The osteoblast: A sophisticated fibroblast under central surveillance. Science 2000, 289, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, F.; Nishimura, R.; Matsubara, T.; Tanaka, S.; Inoue, J.; Reddy, S.V.; Hata, K.; Yamashita, K.; Hiraga, T.; Watanabe, T.; et al. Critical roles of c-jun signaling in regulation of nfat family and rankl-regulated osteoclast differentiation. J. Clin. Investig. 2004, 114, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Iotsova, V.; Caamano, J.; Loy, J.; Yang, Y.; Lewin, A.; Bravo, R. Osteopetrosis in mice lacking nf-kappab1 and nf-kappab2. Nat. Med. 1997, 3, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Yao, Z.; Li, F.; Zhang, Q.; Badell, I.R.; Schwarz, E.M.; Takeshita, S.; Wagner, E.F.; Noda, M.; Matsuo, K.; et al. Nf-kappab p50 and p52 regulate receptor activator of nf-kappab ligand (rankl) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-fos and nfatc1. J. Biol. Chem. 2007, 282, 18245–18253. [Google Scholar] [CrossRef] [PubMed]
- Takatsuna, H.; Asagiri, M.; Kubota, T.; Oka, K.; Osada, T.; Sugiyama, C.; Saito, H.; Aoki, K.; Ohya, K.; Takayanagi, H.; et al. Inhibition of rankl-induced osteoclastogenesis by (-)-dhmeq, a novel nf-kappab inhibitor, through downregulation of nfatc1. J. Bone Miner. Res. 2005, 20, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Nakamura, I.; Jimi, E.; Takahashi, N. Regulation of osteoclast function. J. Bone Miner. Res. 1997, 12, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Vaananen, H.K.; Horton, M. The osteoclast clear zone is a specialized cell-extracellular matrix adhesion structure. J. Cell. Sci. 1995, 108, 2729–2732. [Google Scholar] [PubMed]
- Nishino, T.; Yokoyama, G.; Dobashi, K.; Fujihara, M.; Nagumo, T. Isolation, purification, and characterization of fucose-containing sulfated polysaccharides from the brown seaweed ecklonia kurome and their blood-anticoagulant activities. Carbohydr. Res. 1989, 186, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Dobashi, K.; Nishino, T.; Fujihara, M.; Nagumo, T. Isolation and preliminary characterization of fucose-containing sulfated polysaccharides with blood-anticoagulant activity from the brown seaweed hizikia fusiforme. Carbohydr. Res. 1989, 194, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnicka, F.; Copikova, J.; Park, Y.I. Structure and antitumour activity of fucoidan isolated from sporophyll of korean brown seaweed undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W.L. Fucoidan from New Zealand undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, W.; Takahashi, T.; Kanno, T.; Ichimiya, H.; Shinmyouzu, K.; Takano, H.; Koseki, T.; Nishihara, T. Heparin inhibits osteoclastic differentiation and function. J. Cell. Biochem. 2008, 103, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Shinmyouzu, K.; Takahashi, T.; Ariyoshi, W.; Ichimiya, H.; Kanzaki, S.; Nishihara, T. Dermatan sulfate inhibits osteoclast formation by binding to receptor activator of nf-kappa b ligand. Biochem. Biophys. Res. Commun. 2007, 354, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Salbach, J.; Kliemt, S.; Rauner, M.; Rachner, T.D.; Goettsch, C.; Kalkhof, S.; von Bergen, M.; Moller, S.; Schnabelrauch, M.; Hintze, V.; et al. The effect of the degree of sulfation of glycosaminoglycans on osteoclast function and signaling pathways. Biomaterials 2012, 33, 8418–8429. [Google Scholar] [CrossRef] [PubMed]
- Theoleyre, S.; Kwan Tat, S.; Vusio, P.; Blanchard, F.; Gallagher, J.; Ricard-Blum, S.; Fortun, Y.; Padrines, M.; Redini, F.; Heymann, D. Characterization of osteoprotegerin binding to glycosaminoglycans by surface plasmon resonance: Role in the interactions with receptor activator of nuclear factor kappab ligand (rankl) and rank. Biochem. Biophys. Res. Commun. 2006, 347, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Sudo, T.; Saito, T.; Osada, H.; Tsujimoto, M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of nf-kappa b ligand (rankl). J. Biol. Chem. 2000, 275, 31155–31161. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.G.; Hong, J.M.; Park, J.Y.; Ha, M.H.; Kim, T.H.; Cho, J.Y.; Ryoo, H.M.; Choi, J.Y.; Shin, H.I.; Chun, S.Y.; et al. Proteomic profile of osteoclast membrane proteins: Identification of Na+/H+ exchanger domain containing 2 and its role in osteoclast fusion. Proteomics 2008, 8, 2625–2639. [Google Scholar] [CrossRef] [PubMed]
- Faccio, R.; Takeshita, S.; Zallone, A.; Ross, F.P.; Teitelbaum, S.L. C-fms and the α ν β 3 integrin collaborate during osteoclast differentiation. J. Clin. Investig. 2003, 111, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Battaglino, R.; Kim, D.; Fu, J.; Vaage, B.; Fu, X.Y.; Stashenko, P. C-myc is required for osteoclast differentiation. J. Bone Miner. Res. 2002, 17, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Ninomiya, K.; Fujita, N.; Suzuki, T.; Iwasaki, R.; Morita, K.; Hosogane, N.; Matsuo, K.; Toyama, Y.; Suda, T.; et al. Induction of dc-stamp by alternative activation and downstream signaling mechanisms. J. Bone Miner. Res. 2007, 22, 992–1001. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.W.; Baek, S.-H.; Lee, S.-H.; Kim, T.-H.; Kim, S.-Y. Fucoidan, a Sulfated Polysaccharide, Inhibits Osteoclast Differentiation and Function by Modulating RANKL Signaling. Int. J. Mol. Sci. 2014, 15, 18840-18855. https://doi.org/10.3390/ijms151018840
Kim YW, Baek S-H, Lee S-H, Kim T-H, Kim S-Y. Fucoidan, a Sulfated Polysaccharide, Inhibits Osteoclast Differentiation and Function by Modulating RANKL Signaling. International Journal of Molecular Sciences. 2014; 15(10):18840-18855. https://doi.org/10.3390/ijms151018840
Chicago/Turabian StyleKim, Young Woo, Seung-Hoon Baek, Sang-Han Lee, Tae-Ho Kim, and Shin-Yoon Kim. 2014. "Fucoidan, a Sulfated Polysaccharide, Inhibits Osteoclast Differentiation and Function by Modulating RANKL Signaling" International Journal of Molecular Sciences 15, no. 10: 18840-18855. https://doi.org/10.3390/ijms151018840
APA StyleKim, Y. W., Baek, S. -H., Lee, S. -H., Kim, T. -H., & Kim, S. -Y. (2014). Fucoidan, a Sulfated Polysaccharide, Inhibits Osteoclast Differentiation and Function by Modulating RANKL Signaling. International Journal of Molecular Sciences, 15(10), 18840-18855. https://doi.org/10.3390/ijms151018840