Evaluation of Posterolateral Lumbar Fusion in Sheep Using Mineral Scaffolds Seeded with Cultured Bone Marrow Cells
Abstract
:1. Introduction
2. Results
2.1. In Vivo Ectopic Bone Formation Assay
2.2. Noninvasive Bioluminescence Imaging (BLI) Monitoring of Osteogenic Differentiation and Spinal Fusion of Sheep MSCs Seeded in HA Scaffolds in Nude Mice


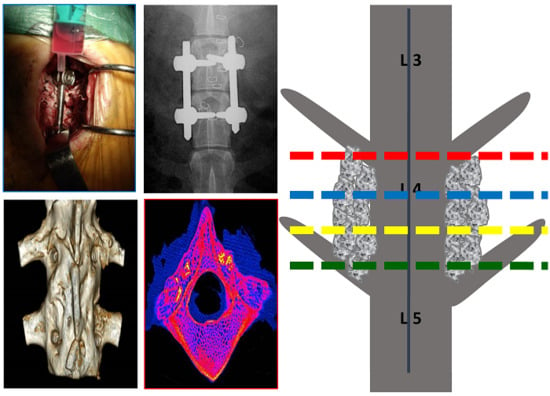
2.3. Spinal Fusion Rates

2.4. Histology



2.5. Histomorphometry

3. Discussion
4. Experimental Section
4.1. Experimental Groups
4.2. Surgical Procedure
4.3. Osteogenic Preparations
4.4. Diagnosis Studies
4.5. Computed Tomography (CT) Scan
4.6. Histology
4.7. Histomorphometry
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Evans, N.R.; Davies, E.M.; Dare, C.J.; Oreffo, R.O. Tissue engineering strategies in spinal arthrodesis: The clinical imperative and challenges to clinical translation. Regen. Med. 2013, 8, 49–64. [Google Scholar] [PubMed]
- Biyani, A.; Andersson, G.B. Low back pain: Pathophysiology and management. J. Am. Acad. Orthop. Surg. 2004, 12, 106–115. [Google Scholar]
- Morone, M.A.; Boden, S.D. Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel. Spine 1998, 23, 159–167. [Google Scholar] [PubMed]
- Slosar, P.J.; Josey, R.; Reynolds, J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: A prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Thalgott, J.S.; Fogarty, M.E.; Giuffre, J.M.; Christenson, S.D.; Epstein, A.K.; Aprill, C. A prospective, randomized, blinded, single-site study to evaluate the clinical and radiographic differences between frozen and freeze-dried allograft when used as part of a circumferential anterior lumbar interbody fusion procedure. Spine 2009, 34, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Pneumaticos, S.G.; Triantafyllopoulos, G.K.; Chatziioannou, S.; Basdra, E.K.; Papavassiliou, A.G. Biomolecular strategies of bone augmentation in spinal surgery. Trends Mol. Med. 2010, 17, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.C.; Herkowitz, H.N. Pseudarthrosis of the spine. Clin. Orthop. Relat. Res. 1992, 284, 80–90. [Google Scholar] [PubMed]
- Reid, J.J.; Johnson, J.S.; Wang, J.C. Challenges to bone formation in spinal fusion. J. Biomech. 2011, 44, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, G.; Cornett, C.A. Bone graft and bone graft substitutes in spine surgery: Current concepts and controversies. J. Am. Acad. Orthop. Surg. 2013, 21, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kruyt, M.C.; van Gaalen, S.M.; Oner, F.C.; Verbout, A.J.; de Bruijn, J.D.; Dhert, W.J. Bone tissue engineering and spinal fusion: The potential of hybrid constructs by combining osteoprogenitor cells and scaffolds. Biomaterials 2004, 25, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Shamsull, B.S.; Tan, K.K.; Chen, H.C.; Aminuddin, B.S.; Ruszymah, B.H. Posterolateral spinal fusion with osteogenesis induced BMSC seeded TCP/HA in a sheep model. Tissue Cell 2014, 46, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Bridwell, K.H.; Anderson, P.A.; Boden, S.D.; Vaccaro, A.R.; Wang, J.C. What’s new in spine surgery. J. Bone Jt. Surg. Am. 2014, 96, 1048–1054. [Google Scholar] [CrossRef]
- Grauer, J.N.; Beiner, J.M.; Kwon, B.; Vaccaro, A.R. The evolution of allograft bone for spinal applications. Orthopedics 2005, 28, 573–577. [Google Scholar] [PubMed]
- Mroz, T.E.; Joyce, M.J.; Steinmetz, M.P.; Lieberman, I.H.; Wang, J.C. Musculoskeletal allograft risks and recalls in the United States. J. Am. Acad. Orthop. Surg. 2008, 16, 559–565. [Google Scholar] [PubMed]
- Tilkeridis, K.; Touzopoulos, P.; Ververidis, A.; Christodoulou, S.; Kazakos, K.; Drosos, G.I. Use of demineralized bone matrix in spinal fusion. World J. Orthop. 2014, 5, 30–37. [Google Scholar] [CrossRef]
- Rihn, J.A.; Makda, J.; Hong, J.; Patel, R.; Hilibrand, A.S.; Anderson, D.G.; Vaccaro, A.R.; Albert, T.J. The use of RhBMP-2 in single-level transforaminal lumbar interbody fusion: A clinical and radiographic analysis. Eur. Spine J. 2009, 18, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.; Bittar, R.G. BMP-7 (OP-1) safety in anterior cervical fusion surgery. J. Clin. Neurosci. 2009, 16, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Dang, G.; Guo, Z.; Yang, M. Evaluation of autologous bone marrow mesenchymal stem cell-calcium phosphate ceramic composite for lumbar fusion in rhesus monkey interbody fusion model. Tissue Eng. 2005, 11, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, O.N.; Dailey, A.T. Mesenchymal stem cells and gene therapies for spinal fusion. Neurosurgery 2008, 63, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Tsumura, H.; Wang, J.C.; Alanay, A. An update on bone substitutes for spinal fusion. Eur. Spine J. 2009, 18, 783–799. [Google Scholar] [CrossRef] [PubMed]
- Garrison, K.R.; Donell, S.; Ryder, J.; Shemilt, I.; Mugford, M.; Harvey, I.; Song, F. Clinical effectiveness and cost effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol. Assess. 2007, 11, 1–150. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.K.; Wang, J.C. The use of bone morphogenetic protein in spine fusion. Spine J. 2008, 8, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Selph, Sh.; McDonagh, M.; Peterson, K.; Tiwari, A.; Chou, R.; Helfand, M. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion. A systematic review and meta-analysis. Ann. Intern. Med. 2013, 158, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Zuk, P.A.; Zou, J.; Yoon, S.H.; Wei, F.; Morishita, Y.; Sintuu, C.; Wang, J.C. Comparison of human mesenchymal stem cells derived from adipose tissue and bone marrow for ex vivo gene therapy in rat spinal fusion model. Spine 2008, 33, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.C.; Li, X.; Shen, F.H. Stem cells in preclinical spine studies. Spine J. 2014, 14, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Lane, J.M. Spinal fusion surgery: Animal models for tissue engineered bone constructs. Biomaterials 2004, 25, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.C.; Theerajunyaporn, T.; Maitra, S. Efficacy of mesenchymal stem cell enriched grafts in an ovine posterolateral lumbar spine model. Spine 2007, 32, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Abbah, S.A.; Lam, C.X.; Ramruttun, K.A.; Goh, J.C.; Wong, H.K. Autogenous bone marrow stromal cell sheets loaded mPCL/TCP scaffolds induced osteogenesis in a porcine model of spinal interbody fusion. Tissue Eng. A 2011, 17, 809–817. [Google Scholar] [CrossRef]
- Andrades, J.A.; Han, B.; Becerra, J.; Sorgente, N.; Hall, F.L.; Nimni, M.E. A recombinant human TGF-beta1 fusion protein with collagen-binding domain promotes migration, growth, and differentiation of bone marrow mesenchymal cells. Exp. Cell Res. 1999, 250, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.; Guerado, E.; Claros, S.; Alonso, M.; Bertrand, M.L.; Gonzalez, C.; Andrades, J.A. Autologous human-derived bone marrow cells exposed to a novel TGF-β1 fusion protein for treatment of critically sized tibial defect. Regen. Med. 2006, 1, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Locklin, R.M.; Williamson, M.C.; Beresford, J.N.; Triffitt, J.T.; Owen, M.E. In vitro effects of growth factors and dexamethasone on rat marrow stromal cells. Clin. Orthop. Relat. Res. 1995, 313, 27–35. [Google Scholar] [PubMed]
- Zimmermann, B.; Wachtel, H.C.; Vormann, J. Kinetics of beta-glycerophosphate-induced endochondral mineralization in vitro. Calcium accumulation, alkaline phosphatase activity, and effects of levamisole. Calcif. Tissue Int. 1992, 51, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Malaval, L.; Modrowski, D.; Gupta, A.K.; Aubin, J.E. Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J. Cell Physiol. 1994, 158, 555–572. [Google Scholar] [CrossRef] [PubMed]
- Giannicola, G.; Ferrari, E.; Citro, G.; Sacchetti, B.; Corsi, A.; Riminucci, M.; Cinotti, G.; Bianco, P. Graft vascularization is a critical rate-limiting step in skeletal stem cell mediated posterolateral spinal fusion. J. Tissue Eng. Regen. Med. 2010, 4, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Claros, S.; Rodríguez-Losada, N.; Cruz, E.; Guerado, E.; Becerra, J.; Andrades, J.A. Characterization of adult stem/progenitor cell populations from bone marrow in a three dimensional collagen gel culture system. Cell Transplant. 2012, 21, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cell Mater. 2008, 1, 53–76. [Google Scholar]
- Curylo, L.J.; Johnstone, B.; Petersilge, C.A.; Janicki, J.A.; Yoo, J.U. Augmentation of spinal arthrodesis with autologous bone marrow in a rabbit posterolateral spine fusion model. Spine 1999, 24, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Boyde, A.; Corsi, A.; Quarto, R.; Cancedda, R.; Bianco, P. Osteoconduction in large macroporous hydroxyapatite ceramic implants: evidence for a complementary integration and disintegration mechanism. Bone 1999, 24, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Kruyt, M.C.; de Bruijn, J.D.; Wilson, C.E.; Oner, F.C.; van Blitterswijk, C.A.; Verbout, A.J.; Dhert, W.J. Viable osteogenic cells are obligatory for tissue-engineered ectopic bone formation in goats. Tissue Eng. A 2003, 9, 327–336. [Google Scholar] [CrossRef]
- Granero-Moltó, F.; Weis, J.A.; Miga, M.I. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.; Santos-Ruiz, L.; Andrades, J.A. The stem cell niche should be a key issue for cell therapy in regenerative medicine. Stem Cell Rev. 2010, 7, 248–255. [Google Scholar] [CrossRef]
- Walsh, W.R.; Oliver, R.A.; Gage, G. Application of resorbable poly(lactide-coglycolide) with entangled hyaluronic acid as an autograft extender for posterolateral intertransverse lumbar fusion in rabbits. Tissue Eng. A 2011, 17, 213–220. [Google Scholar] [CrossRef]
- Rozen, N.; Bick, T.; Bajayo, A.; Shamian, B.; Schrift-Tzadok, M.; Gabet, Y.; Yayon, A.; Bab, I.; Soudry, M.; Lewinson, D. Transplanted blood-derived endothelial progenitor cells (EPC) enhance bridging of sheep tibia critical size defects. Bone 2009, 45, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocker, A.K.; Woodward, H.N.; Hall, B.K. The biology of bone. In Bone Histology of Fossil Tetrapods; Padian, K., Lamm, E.T., Eds.; University of California Press: Berkeley, CA, USA, 2013; pp. 13–34. [Google Scholar]
- Andrades, J.A.; Santamaría, J.A.; Nimni, M.E.; Becerra, J. Selection and amplification of a bone marrow cell population and its induction to the chondro-osteogenic lineage by rhOP-1: An in vitro and in vivo study. Int. J. Dev. Biol. 2001, 45, 689–693. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuenca-López, M.D.; Andrades, J.A.; Gómez, S.; Zamora-Navas, P.; Guerado, E.; Rubio, N.; Blanco, J.; Becerra, J. Evaluation of Posterolateral Lumbar Fusion in Sheep Using Mineral Scaffolds Seeded with Cultured Bone Marrow Cells. Int. J. Mol. Sci. 2014, 15, 23359-23376. https://doi.org/10.3390/ijms151223359
Cuenca-López MD, Andrades JA, Gómez S, Zamora-Navas P, Guerado E, Rubio N, Blanco J, Becerra J. Evaluation of Posterolateral Lumbar Fusion in Sheep Using Mineral Scaffolds Seeded with Cultured Bone Marrow Cells. International Journal of Molecular Sciences. 2014; 15(12):23359-23376. https://doi.org/10.3390/ijms151223359
Chicago/Turabian StyleCuenca-López, María D., José A. Andrades, Santiago Gómez, Plácido Zamora-Navas, Enrique Guerado, Nuria Rubio, Jerónimo Blanco, and José Becerra. 2014. "Evaluation of Posterolateral Lumbar Fusion in Sheep Using Mineral Scaffolds Seeded with Cultured Bone Marrow Cells" International Journal of Molecular Sciences 15, no. 12: 23359-23376. https://doi.org/10.3390/ijms151223359
APA StyleCuenca-López, M. D., Andrades, J. A., Gómez, S., Zamora-Navas, P., Guerado, E., Rubio, N., Blanco, J., & Becerra, J. (2014). Evaluation of Posterolateral Lumbar Fusion in Sheep Using Mineral Scaffolds Seeded with Cultured Bone Marrow Cells. International Journal of Molecular Sciences, 15(12), 23359-23376. https://doi.org/10.3390/ijms151223359