Differential Effects of High-Fish Oil and High-Lard Diets on Cells and Cytokines Involved in the Inflammatory Process in Rat Insulin-Sensitive Tissues
Abstract
:1. Introduction
2. Results and Discussion
2.1. Body Weight Gain and Energy Intake
2.2. Serum Metabolite, Hormone and Adipokine Levels
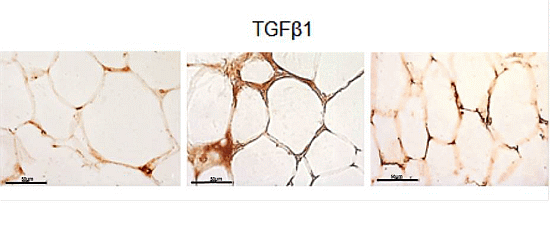
2.3. eWAT Histology and Immunohistochemistry
2.4. Skeletal Muscle Fibre Histology and Immunohistochemistry
2.5. Hepatic Steatosis
3. Experimental Section
3.1. Materials
3.2. Animals and Diets
3.3. Determination of Serum Parameters
3.4. Histology
3.5. Determination of Adipocyte Size Using Computer Image Analysis
3.6. Immunohistochemistry
3.7. Preparation of Skeletal Muscle and Liver Extract
3.8. Lipid and TNF-α Content
3.9. Electrophoresis and Western Blot Analysis
3.10. Data Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wellen, K.E.; Hotamisligil, G.S. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Invest 2003, 112, 1785–1788. [Google Scholar]
- Lionetti, L.; Mollica, M.P.; Lombardi, A.; Cavaliere, G.; Gifuni, G.; Barletta, A. From chronic overnutrition to insulin resistance: The role of fat-storing capacity and inflammation. Nutr. Metab. Cardiovasc. Dis 2009, 19, 146–152. [Google Scholar]
- Mollica, M.P.; Lionetti, L.; Putti, R.; Cavaliere, G.; Gaita, M.; Barletta, A. From chronic overfeeding to hepatic injury: Role of endoplasmic reticulum stress and inflammation. Nutr. Metab. Cardiovasc. Dis 2011, 21, 222–230. [Google Scholar]
- Sethi, J.K.; Vidal-Puig, A.J. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lip. Res 2007, 48, 1253–1262. [Google Scholar]
- Abete, I.; Goyenechea, E.; Zulet, M.A.; Martínez, J.A. Obesity and metabolic syndrome: Potential benefit from specific nutritional components. Nutr. Metab. Cardiovasc. Dis 2011, 21, B1–B15. [Google Scholar]
- Gonzalez-Periz, A.; Horrillo, R.; Ferre, N.; Gronert, K.; Dong, B.; Morán-Salvador, E.; Titos, E.; Martínez-Clemente, M.; López-Parra, M.; Arroyo, V.; et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: A role for resolvins and protectins. FASEB J 2009, 23, 1946–1957. [Google Scholar]
- Nakatani, T.; Kim, H.J.; Kaburagi, Y.; Yasuda, K.; Ezaki, O. A low fish oil inhibits SREBP-1 proteolytic cascade, while a high-fish-oil feeding decreases SREBP-1 mRNA in mice liver: Relationship to anti-obesity. J. Lip. Res 2003, 44, 369–379. [Google Scholar]
- Xin, Y.N.; Xuan, S.Y.; Zhang, J.H.; Zheng, M.H.; Guan, H.S. Omega-3 polyunsaturated fatty acids: A specific liver drug for non-alcoholic fatty liver disease (NAFLD). Med. Hypotheses 2008, 71, 820–821. [Google Scholar]
- Calder, P.C. Long-chain fatty acids and inflammation. Proc. Natl. Acad. Sci 2012, 71, 284–289. [Google Scholar]
- Kalupahana, N.S.; Claycombe, K.J.; Moustaid-Moussa, N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv. Nutr 2011, 2, 304–316. [Google Scholar]
- Saidpour, A.; Zahediasl, S.; Kimiagar, M.; Vafa, M.; Ghasemi, A.; Abadi, A.; Daneshpour, M.S.; Zarkesh, M. Fish oil and olive oil can modify insulin resistance and plasma desacyl-ghrelin in rats. J. Res. Med. Sci 2011, 16, 862–871. [Google Scholar]
- Storlien, L.H.; Kraegen, E.W.; Chisholm, D.J.; Ford, G.L.; Bruce, D.G.; Pascoe, W.S. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 1987, 237, 885–888. [Google Scholar]
- Ghafoorunissa, I.A.; Rajkumar, L.; Acharya, V. Dietary (n-3) long chain polyunsaturated fatty acids prevent sucrose-induced insulin resistance in rats. J. Nutr 2005, 135, 2634–2638. [Google Scholar]
- Flachs, P.; Mohamed-Ali, V.; Horakova, O.; Rossmeisl, M.; Hosseinzadeh-Attar, M.J.; Hensler, M.; Ruzickova, J.; Kopecky, J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia 2006, 49, 394–397. [Google Scholar]
- Saraswathi, V.; Morrow, J.D.; Hasty, A.H. Dietary fish oil exerts hypolipidemic effects in lean and insulin sensitizing effects in obese LDLR−/− mice. J. Nutr 2009, 139, 2380–2386. [Google Scholar]
- Kalupahana, N.S.; Claycombe, K.; Newman, S.; Stewart, T.; Siriwardhana, N.; Matthan, N.; Lichtenstein, A.H.; Moustaid-Moussa, N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J. Nutr 2010, 140, 1915–1922. [Google Scholar]
- Lionetti, L.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Gifuni, G.; Gaita, M.; Pignalosa, A.; Donizzetti, I.; Putti, R.; Barletta, A. Effects of endoplasmic reticulum (ER) stress on insulin action and mitochondrial energy efficiency in skeletal muscle of rats fed with lard or fish oil. Obesity Facts 2012, 5. [Google Scholar]
- Lionetti, L.; Mollica, M.P.; Sica, R.; Donizzetti, I.; Gifuni, G.; Pignalosa, A.; Cavaliere, G.; Putti, R. Hepatic mitochondrial bioenergetic and dynamic behaviour adaptations in response to high-fat feeding. BBA Bioenergetics 2012, 1817, S73. [Google Scholar]
- Murata, M.; Kaji, H.; Takahashi, Y.; Iida, K.; Mizuno, I.; Okimura, Y.; Abe, H.; Chihara, K. Stimulation by eicosapentaenoic acids of leptin mRNA expression and its secretion in mouse 3T3-L1 adipocytes in vitro. Biochem. Biophys. Res. Commun. 2000, 270, 343–348. [Google Scholar]
- Pérez-Matute, P.; Marti, A.; Martínez, J.A.; Fernández-Otero, M.P.; Stanhope, K.L.; Havel, P.J.; Moreno-Aliaga, M.J. Eicosapentaenoic fatty acid increases leptin secretion from primary cultured rat adipocytes: Role of glucose metabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol 2005, 288, R1682–R1688. [Google Scholar]
- Reseland, J.E.; Haugen, F.; Hollung, K.; Solvoll, K.; Halvorsen, B.; Brude, I.R.; Nenseter, M.S.; Christiansen, E.N.; Drevon, C.A. Reduction of leptin gene expression by dietary polyunsaturated fatty acids. J. Lip. Res 2001, 42, 743–750. [Google Scholar]
- Tishinsky, J.M.; Ma, D.W.; Robinson, L.E. Eicosapentaenoic acid and rosiglitazone increase adiponectin in an additive and PPARγ-dependent manner in human adipocytes. Obesity 2011, 19, 262–268. [Google Scholar]
- Luo, J.; Rizkalla, S.W.; Boillot, J.; Alamowitch, C.; Chaib, H.; Bruzzo, F.; Desplanque, N.; Dalix, A.M.; Durand, G.; Slama, G. Dietary (n-3) polyunsaturated fatty acids improve adipocyte insulin action and glucose metabolism in insulin-resistant rats: relation to membrane fatty acids. J. Nutr 1996, 126, 1951–1958. [Google Scholar]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lip. Res 2005, 46, 2347–2355. [Google Scholar]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest 2003, 112, 1796–1808. [Google Scholar]
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci 2003, 100, 7265–7270. [Google Scholar]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest 2006, 116, 1494–1505. [Google Scholar]
- Matsushima, K.; Larsen, C.G.; DuBois, G.C.; Oppenheim, J.J. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J. Exp. Med 1989, 169, 1485–1490. [Google Scholar]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest 2003, 112, 1821–1830. [Google Scholar]
- Samad, F.; Yamamoto, K.; Pandey, M.; Loskutoff, D.J. Elevated expression of transforming growth factor-β in adipose tissue from obese mice. Mol. Med 1997, 3, 37–48. [Google Scholar]
- Sciarretta, S.; Ferrucci, A.; Ciavarella, G.M.; De Paolis, P.; Venturelli, V.; Tocci, G.; De Biase, L.; Rubattu, S.; Volpe, M. Markers of inflammation and fibrosis are related to cardiovascular damage in hypertensive patients with metabolic syndrome. Am. J. Hypert 2007, 20, 784–791. [Google Scholar]
- Tan, C.K.; Chong, H.C.; Tan, E.H.; Tan, N.S. Getting “Smad” about obesity and diabetes. Nutr. Diabetes 2012, 2, e29–e35. [Google Scholar]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest 2007, 117, 175–184. [Google Scholar]
- Fujisaka, S.; Usui, I.; Bukhari, A.; Ikutani, M.; Oya, T.; Kanatani, Y.; Tsuneyama, K.; Nagai, Y.; Takatsu, K.; Urakaze, M.; et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009, 58, 2574–2582. [Google Scholar]
- Figueras, M.; Olivan, M.; Busquets, S.; López-Soriano, F.J.; Argilés, J.M. Effects of eicosapentaenoic acid (EPA) treatment on insulin sensitivity in an animal model of diabetes: improvement of the inflammatory status. Obesity 2011, 19, 362–369. [Google Scholar]
- Hendrich, S. (n-3) Fatty acids: Clinical trials in people with type 2 diabetes. Adv. Nutr 2010, 1, 3–7. [Google Scholar]
- Puglisi, M.J.; Hasty, A.H.; Saraswathi, V. The role of adipose tissue in mediating the beneficial effects of dietary fish oil. J. Nutr. Biochem 2011, 22, 101–108. [Google Scholar]
- Auwerx, J. PPARgamma, the ultimate thrifty gene. Diabetologia 1999, 42, 1033–1049. [Google Scholar]
- Banga, A.; Unal, R.; Tripathi, P.; Pokrovskaya, I.; Owens, R.J.; Kern, P.A.; Ranganathan, G. Adiponectin translation is increased by the PPARgamma agonists pioglitazone and omega-3 fatty acids. Am. J. Physiol. Endocrinol. MeTable 2009, 296, E480–E489. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem 1957, 226, 497–510. [Google Scholar]
- Sato, S.; Hughes, C. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J. Biol. Chem 1994, 269, 4424–4430. [Google Scholar]
- Li, P.; Lu, M.; Nguyen, M.T.; Bae, E.J.; Chapman, J.; Feng, D.; Hawkins, M.; Pessin, J.E.; Sears, D.D.; Nguyen, A.K.; et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J. Biol. Chem 2010, 285, 15333–15345. [Google Scholar]
- Spencer, M.; Yao-Borengasser, A.; Unal, R.; Rasouli, N.; Gurley, C.M.; Zhu, B.; Peterson, C.A.; Kern, P.A. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen IV and fibrosis and demonstrate alternative activation. Am. J. Physiol 2010, 299, E1016–E1027. [Google Scholar]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Invest 2011, 121, 2094–2101. [Google Scholar]
- Lionetti, L.; Sica, R.; Mollica, M.P.; Putti, R. High-lard and high-fish oil diets differ in their effects on insulin resistance development, mitochondrial morphology and dynamic behaviour in rat skeletal muscle. Food Nutr. Sci 2013, 4, 105–112. [Google Scholar]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med 2002, 8, 1288–1295. [Google Scholar]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Poirier, F.; Russo, F.P.; Iredale, J.P.; Haslett, C.; Simpson, K.J.; Sethi, T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci 2006, 103, 5060–5065. [Google Scholar]
- Gaca, M.D. Regulation of hepatic stellate cell proliferation and collagen synthesis by proteinase-activated. J. Hepat 2002, 36, 362–369. [Google Scholar]
- Sarem, M.; Znaidak, R.; Macías, M.; Rey, R. Hepatic stellate cell: It is role in normal and pathological conditions. Gastroenter. Hepat 2006, 29, 93–101. [Google Scholar]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar]
- Long, Y.C.; Zierath, J.R. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Invest 2006, 116, 1776–1783. [Google Scholar]
- Iossa, S.; Lionetti, L.; Mollica, M.P.; Crescenzo, R.; Botta, M.; Barletta, A.; Liverini, G. Effect of high-fat feeding on metabolic efficiency and mitochondrial oxidative capacity in adult rats. Br. J. Nutr 2003, 90, 953–960. [Google Scholar] [Green Version]
- De Lange, P.; Cioffi, F.; Senese, R.; Moreno, M.; Lombardi, A.; Silvestri, E.; De Matteis, R.; Lionetti, L.; Mollica, M.P.; Goglia, F.; et al. Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-l-thyronine in rats. Diabetes 2011, 60, 2730–2739. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Towbin, H.; Staehelintl, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci 1979, 76, 4350–4354. [Google Scholar]










| N | L | F | |
|---|---|---|---|
| Body-weight gain, g/week | 16.8 ± 1.0 | 29.7 ± 3.1 * | 21.5 ± 1.9 # |
| Food intake, g/week | 136.4 ± 10.1 | 167.5 ± 9.2 * | 168.3 ± 8.3 * |
| Feeding efficiency, % | 12.3 ± 1.1 | 17.7 ± 1.5 * | 12.8 ± 1.0 # |
| Retroperitoneal WAT, g | 7.05 ± 0.31 | 16.27 ± 0.73 * | 9.8 ± 0.51 *# |
| Epididymal WAT, g | 7.97 ± 0.28 | 14.6 ± 1.6 * | 11.52 ± 0.61 *# |
| Component | Control Diet | High-fat diet | |
|---|---|---|---|
| High-Lard g/100 g diet | High-Fish Oil g/100 g diet | ||
| Standard feed g | 100 | 51.03 | 51.03 |
| Casein a g | - | 9.25 | 9.25 |
| Lard g | - | 21.8 | - |
| Fish oil b g | - | - | 21.8 |
| Sunflower oil g | - | 1.24 | 1.24 |
| AIN 76 Mineral mix c g | - | 1.46 | 1.46 |
| AIN 76 Vitamin mix d g | - | 0.42 | 0.42 |
| Choline bitartrate g | - | 0.08 | 0.08 |
| Methionine g | - | 0.12 | 0.12 |
| Energy density, kJ/g diet | 15.88 | 20.00 | 20.00 |
| Energy (J/100 J) | |||
| Protein% | 29 | 29 | 29 |
| Lipid% | 10.6 | 40 | 40 |
| Carbohydrate% | 60.4 | 31 | 31 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lionetti, L.; Mollica, M.P.; Sica, R.; Donizzetti, I.; Gifuni, G.; Pignalosa, A.; Cavaliere, G.; Putti, R. Differential Effects of High-Fish Oil and High-Lard Diets on Cells and Cytokines Involved in the Inflammatory Process in Rat Insulin-Sensitive Tissues. Int. J. Mol. Sci. 2014, 15, 3040-3063. https://doi.org/10.3390/ijms15023040
Lionetti L, Mollica MP, Sica R, Donizzetti I, Gifuni G, Pignalosa A, Cavaliere G, Putti R. Differential Effects of High-Fish Oil and High-Lard Diets on Cells and Cytokines Involved in the Inflammatory Process in Rat Insulin-Sensitive Tissues. International Journal of Molecular Sciences. 2014; 15(2):3040-3063. https://doi.org/10.3390/ijms15023040
Chicago/Turabian StyleLionetti, Lillà, Maria Pina Mollica, Raffaella Sica, Immacolata Donizzetti, Giorgio Gifuni, Angelica Pignalosa, Gina Cavaliere, and Rosalba Putti. 2014. "Differential Effects of High-Fish Oil and High-Lard Diets on Cells and Cytokines Involved in the Inflammatory Process in Rat Insulin-Sensitive Tissues" International Journal of Molecular Sciences 15, no. 2: 3040-3063. https://doi.org/10.3390/ijms15023040
APA StyleLionetti, L., Mollica, M. P., Sica, R., Donizzetti, I., Gifuni, G., Pignalosa, A., Cavaliere, G., & Putti, R. (2014). Differential Effects of High-Fish Oil and High-Lard Diets on Cells and Cytokines Involved in the Inflammatory Process in Rat Insulin-Sensitive Tissues. International Journal of Molecular Sciences, 15(2), 3040-3063. https://doi.org/10.3390/ijms15023040