Inhibitory Effects of Palmultang on Inflammatory Mediator Production Related to Suppression of NF-κB and MAPK Pathways and Induction of HO-1 Expression in Macrophages
Abstract
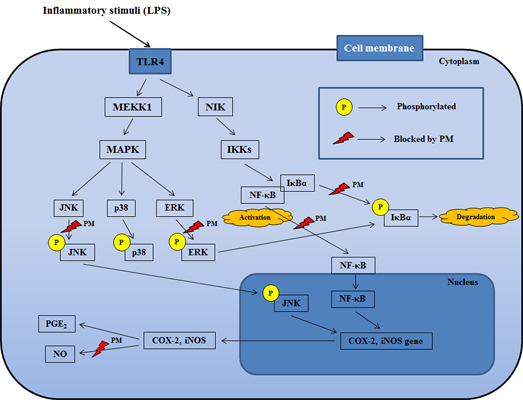
:1. Introduction
2. Results and Discussion
2.1. PM Did not Show Cytotoxicity and Had Inhibitory Activity against NO and Inflammatory Cytokine Production in Macrophages
2.2. PM Strongly Suppresses Expression of iNOS but not COX-2 in LPS-Stimulated Macrophages and Induces HO-1 Induction
2.3. PM Inhibited NF-κB Pathway Activation via Blockade of IκBα Degradation in Macrophages upon LPS Stimulation
2.4. PM Suppressed LPS-Induced Phosphorylation of MAPKs in RAW 264.7 Cells
2.5. HPLC Analysis and Previous Reports on the Main Constituents of PM
3. Experimental Section
3.1. Materials and Reagents
3.2. Preparation of PM Extract
3.3. Cell Culture and Drug Treatment
3.4. Cell Viability Assay
3.5. Determination of NO, TNF-α, IL-6 and IL-1β Cytokine Production
3.6. Preparation of Whole-Cell, Cytosolic and Nuclear Fractions and Western Blot Analysis
3.7. RNA Extraction and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3.8. Preparation of Standard Solutions and Samples
3.9. General Experimental Procedures
3.10. Analytical Chromatographic Conditions
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lee, M.J.; Lim, E.M.; Kwon, K.R. Effect of Paljin-tang on Surgically Induced Endometriosis in Rats. J. Orient. Obstet. Gynecol 2006, 19, 83–94. [Google Scholar]
- Joo, J.M.; Kim, D.C.; Back, S.H.; Kim, E.H. The Effect of Palmultang on the Ovarian Functions and Differential Gene Expression of Caspase-3, MAPK and MPG in Female Mice. J. Orient. Obstet. Gynecol 2007, 20, 91–110. [Google Scholar]
- Pierce, G.F. Macrophages: Important physiologic and pathologic sources of polypeptide growth factors. Am. J. Respir. Cell Mol. Biol 1990, 2, 233–234. [Google Scholar]
- Wadleigh, D.J.; Reddy, S.T.; Kopp, E.; Ghosh, S.; Herschman, H.R. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J. Biol. Chem 2000, 275, 6259–6266. [Google Scholar]
- Becker, S.; Mundandhara, S.; Devlin, R.B.; Madden, M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: Further mechanistic studies. Toxicol. Appl. Pharmacol 2005, 207, 269–275. [Google Scholar]
- Kim, J.B.; Han, A.R.; Park, E.Y.; Kim, J.Y.; Cho, W.; Lee, J.; Seo, E.K.; Lee, K.T. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells. Biol. Pharm. Bull 2007, 30, 2345–2351. [Google Scholar]
- Pae, H.O.; Chung, H.T. Heme oxygenase-1: Its therapeutic roles in inflammatory diseases. Immune Netw 2009, 9, 12–19. [Google Scholar]
- Ashino, T.; Yamanaka, R.; Yamamoto, M.; Shimokawa, H.; Sekikawa, K.; Iwakura, Y.; Shioda, S.; Numazawa, S.; Yoshida, T. Negative feedback regulation of lipopolysaccharide-induced inducible nitric oxide synthase gene expression by heme oxygenase-1 induction in macrophages. Mol. Immunol 2008, 45, 2106–2115. [Google Scholar]
- De Martin, R.; Vanhove, B.; Cheng, Q.; Hofer, E.; Csizmadia, V.; Winkler, H.; Bach, F.H. Cytokine-inducible expression in endothelial cells of an I kappa B alpha-like gene is regulated by NF kappa B. EMBO J 1993, 12, 2773–2779. [Google Scholar]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar]
- Baeuerle, P.A.; Baltimore, D. NF-kappa B. Ten years after. Cell 1996, 87, 13–20. [Google Scholar]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol 1997, 9, 180–186. [Google Scholar]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol 2003, 54, 469–487. [Google Scholar]
- Southan, G.J.; Szabo, C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem. Pharmacol 1996, 51, 383–394. [Google Scholar]
- Brasier, A.R. The NF-kappaB regulatory network. Cardiovasc. Toxicol 2006, 6, 111–130. [Google Scholar]
- Gilmore, T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar]
- Tian, B.; Brasier, A.R. Identification of a nuclear factor kappa B-dependent gene network. Recent Prog. Horm. Res 2003, 58, 95–130. [Google Scholar]
- Chen, F.; Kuhn, D.C.; Sun, S.C.; Gaydos, L.J.; Demers, L.M. Dependence and reversal of nitric oxide production on NF-kappa B in silica and lipopolysaccharide-induced macrophages. Biochem. Biophys. Res. Commun 1995, 214, 839–846. [Google Scholar]
- Roshak, A.K.; Jackson, J.R.; McGough, K.; Chabot-Fletcher, M.; Mochan, E.; Marshall, L.A. Manipulation of distinct NFkappaB proteins alters interleukin-1beta-induced human rheumatoid synovial fibroblast prostaglandin E2 formation. J. Biol. Chem 1996, 271, 31496–31501. [Google Scholar]
- Xie, W.; Merrill, J.R.; Bradshaw, W.S.; Simmons, D.L. Structural determination and promoter analysis of the chicken mitogen-inducible prostaglandin G/H synthase gene and genetic mapping of the murine homolog. Arch. Biochem. Biophys 1993, 300, 247–252. [Google Scholar]
- Ahn, K.S.; Noh, E.J.; Zhao, H.L.; Jung, S.H.; Kang, S.S.; Kim, Y.S. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-kappaB activation in RAW 264.7 cells. Life Sci 2005, 76, 2315–2328. [Google Scholar]
- Barnes, P.J.; Karin, M. Nuclear factor-kappaB. A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med 1997, 336, 1066–1071. [Google Scholar]
- Kim, Y.M.; Lee, B.S.; Yi, K.Y.; Paik, S.G. Upstream NF-kappaB site is required for the maximal expression of mouse inducible nitric oxide synthase gene in interferon-gamma plus lipopolysaccharide induced RAW 264.7 macrophages. Biochem. Pharmacol 1997, 236, 655–660. [Google Scholar]
- Caivano, M. Role of MAP kinase cascades in inducing arginine transporters and nitric oxide synthetase in RAW 264.7 macrophages. FEBS Lett 1999, 429, 249–253. [Google Scholar]
- Kim, H.K.; Choi, Y.W.; Lee, E.N.; Park, J.K.; Kim, S.G.; Park, D.J.; Kim, B.S.; Lim, Y.T.; Yoon, S. 5-Hydroxymethylfurfural from black garlic extract prevents TNFα-induced monocytic cell adhesion to HUVECs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-κB activation. Phytother. Res 2011, 25, 965–974. [Google Scholar]
- Chen, T.; Guo, Z.P.; Jiao, X.Y.; Jia, R.Z.; Zhang, Y.H.; Li, J.Y.; Huang, X.L.; Liu, H.J. Peoniflorin suppresses tumor necrosis factor-α induced chemokine production in human dermal microvascular endothelial cells by blocking nuclear factor-κB and ERK pathway. Arch. Dermatol. Res 2011, 303, 351–360. [Google Scholar]
- Rim, H.K.; Cho, W.; Sung, S.H.; Lee, K.T. Nodakenin suppresses lipopolysaccharide-induced inflammatory responses in macrophage cells by inhibiting tumor necrosis factor receptor-associated factor 6 and nuclear factor-κB pathways and protects mice from lethal endotoxin shock. J. Pharmacol. Exp. Ther 2012, 342, 654–664. [Google Scholar]
- Kato, T.; Horie, N.; Hashimoto, K.; Satoh, K.; Shimoyama, T.; Kaneko, T.; Kusama, K.; Sakagami, H. Bimodal effect of glycyrrhizin on macrophage nitric oxide and prostaglandin E2 production. In Vivo 2008, 22, 583–586. [Google Scholar]
- Kim, J.H.; Jeong, J.H.; Jeon, S.T.; Kim, H.; Ock, J.; Suk, K.; Kim, S.I.; Song, K.S.; Lee, W.H. Decursin inhibits induction of inflammatory mediators by blocking nuclear factor-kappaB activation in macrophages. Mol. Pharmacol 2006, 69, 1783–1790. [Google Scholar]
- Choi, H.J.; Kang, O.H.; Park, P.S.; Chae, H.S.; Oh, Y.C.; Lee, Y.S.; Choi, J.G.; Lee, G.H.; Kweon, O.H.; Kwon, D.Y. Mume Fructus water extract inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated macrophages. J. Med. Food 2007, 10, 460–466. [Google Scholar]
- Jo, H.Y.; Kim, Y.; Nam, S.Y.; Lee, B.J.; Kim, Y.B.; Yun, Y.W.; Ahn, B. The inhibitory effect of quercitrin gallate on iNOS expression induced by lipopolysaccharide in Balb/c mice. J. Vet. Sci 2008, 9, 267–272. [Google Scholar]
- Kim, H.Y.; Kim, J.K.; Choi, J.H.; Jung, J.Y.; Oh, W.Y.; Kim, D.C.; Lee, H.S.; Kim, Y.S.; Kang, S.S.; Lee, S.H.; et al. Hepatoprotective effect of pinoresinol on carbon tetrachloride-induced hepatic damage in mice. J. Pharmacol. Sci 2010, 112, 105–112. [Google Scholar]
- Srisook, K.; Palachot, M.; Mongkol, N.; Srisook, E.; Sarapusit, S. Anti-inflammatory effect of ethyl acetate extract from Cissus quadrangularis Linn may be involved with induction of heme oxygenase-1 and suppression of NF-κB activation. J. Ethnopharmacol 2011, 133, 1008–1014. [Google Scholar]
- Kwon, D.J.; Ju, S.M.; Youn, G.S.; Choi, S.Y.; Park, J. Suppression of iNOS and COX-2 expression by flavokawain A via blockade of NF-κB and AP-1 activation in RAW 264.7 macrophages. Food Chem. Toxicol 2013, 58, 479–486. [Google Scholar]






| Compound | Linear Range (μg/mL) | Regression Equation a | Correlation Coefficient (r2) | LOD b (μg/mL) | LOQ c (μg/mL) |
|---|---|---|---|---|---|
| 5-HMF | 1.25–10,000 | y = 403917x − 87212 | 0.9993 | 0.16 | 0.50 |
| Ferulic acid | 1.25–10,000 | y = 273782x + 89791 | 0.9998 | 0.23 | 0.68 |
| Nodakenin | 1.25–10,000 | y = 239585x − 25958 | 1.0000 | 0.13 | 0.40 |
| Decursinol | 1.25–10,000 | y = 598246x + 235041 | 0.9996 | 0.10 | 0.29 |
| Glycyrrhizin | 1.25–10,000 | y = 43887x + 38994 | 0.9991 | 0.63 | 0.19 |
| Decursin | 1.25–20,000 | y = 116410x + 1188529 | 1.0000 | 0.45 | 0.12 |
| Peaoniflorin | 20–20,000 | y = 1871.8x + 16715 | 0.9990 | 16.05 | 48.17 |
| Albiflorin | 20–20,000 | y = 8026.8x + 6805.9 | 0.9993 | 3.33 | 10.00 |
| Compound | Content (mg/g) | ||
|---|---|---|---|
| Mean | SD | RSD (%) | |
| 5-HMF | 11.09 | 0.35 | 3.11 |
| Ferulic acid | 2.59 | 0.00 | 0.15 |
| Nodakenin | 2.30 | 0.00 | 0.02 |
| Decursinol | 3.36 | 0.02 | 0.72 |
| Glycyrrhizin | 8.23 | 0.01 | 0.13 |
| Decursin | 5.11 | 0.00 | 0.01 |
| Peaoniflorin | 0.36 | 0.00 | 0.34 |
| Albiflorin | 1.17 | 0.03 | 2.41 |
| Herbs | Amount of Herbs (g) |
|---|---|
| Ginseng Radix | 240 |
| Atractylodes Rhizome White | 240 |
| Poria | 240 |
| Glycyrrhizae Radix et Rhizoma | 240 |
| Angelica Gigas Root | 240 |
| Prepared Rehmannia Root | 240 |
| Peony Root | 240 |
| Cinidium Rhizome | 240 |
| Total weight | 1920 |
| Target Gene | Primer Sequence | Annealing Temp |
|---|---|---|
| TNF-α | F: 5′-AGCACAGAAAGCATGATCCG-3′ R: 5′-GTTTGCTACGACGTGGGCTA-3′ | 55 °C |
| IL-6 | F: 5′-CATGTTCTCTGGGAAATCGTGG-3′ R: 5′-AACGCACTAGGTTTGCCGAGTA-3′ | 58 °C |
| IL-1β | F: 5′-TGCAGAGTTCCCCAACTGGTACATC-3′ R: 5′-GTGCTGCCTAATGTCCCCTTGAATC-3′ | 64 °C |
| COX-2 | F: 5′-CACTCAGTTTGTTGAGTCATTC-3′ R: 5′-GATTAGTACTGTAGGGTTAATG-3′ | 45 °C |
| iNOS | F: 5′-AGCCCAACAATACAAATGACCCTA-3′ R: 5′-TTCCTGTTGTTTCTATTTCCTTTGT-3′ | 56 °C |
| HO-1 | F: 5′-TGAAGGAGGCCACCAAGGAGG-3′ R: 5′-AGAGGTCACCCAGGTAGCGGG-3′ | 62 °C |
| β-actin | F: 5′-ATGAAGATCCTGACCGAGCGT-3′ R: 5′-AACGCAGCTCAGTAACAGTCCG-3′ | 58 °C |
| Item | Condition | ||
|---|---|---|---|
| Mobile phase | Time (min) | Water (Containing 0.1% TFA) | Acetonitrile |
| 0 | 5 | 95 | |
| 5 | 5 | 95 | |
| 15 | 15 | 85 | |
| 25 | 15 | 85 | |
| 50 | 65 | 35 | |
| 60 | 65 | 35 | |
| Flow rate | 1.0 mL/min | ||
| Inject volume | 20 μL | ||
| Column | OptimaPak C18 (4.6 × 250 mm, 5 μm, RS tech Co., Daejeon, Korea) | ||
| Column temperature | 40 °C | ||
| UV wavelength | 205, 250 and 330 nm | ||
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Oh, Y.-C.; Jeong, Y.H.; Cho, W.-K.; Gu, M.-J.; Ma, J.Y. Inhibitory Effects of Palmultang on Inflammatory Mediator Production Related to Suppression of NF-κB and MAPK Pathways and Induction of HO-1 Expression in Macrophages. Int. J. Mol. Sci. 2014, 15, 8443-8457. https://doi.org/10.3390/ijms15058443
Oh Y-C, Jeong YH, Cho W-K, Gu M-J, Ma JY. Inhibitory Effects of Palmultang on Inflammatory Mediator Production Related to Suppression of NF-κB and MAPK Pathways and Induction of HO-1 Expression in Macrophages. International Journal of Molecular Sciences. 2014; 15(5):8443-8457. https://doi.org/10.3390/ijms15058443
Chicago/Turabian StyleOh, You-Chang, Yun Hee Jeong, Won-Kyung Cho, Min-Jung Gu, and Jin Yeul Ma. 2014. "Inhibitory Effects of Palmultang on Inflammatory Mediator Production Related to Suppression of NF-κB and MAPK Pathways and Induction of HO-1 Expression in Macrophages" International Journal of Molecular Sciences 15, no. 5: 8443-8457. https://doi.org/10.3390/ijms15058443
APA StyleOh, Y. -C., Jeong, Y. H., Cho, W. -K., Gu, M. -J., & Ma, J. Y. (2014). Inhibitory Effects of Palmultang on Inflammatory Mediator Production Related to Suppression of NF-κB and MAPK Pathways and Induction of HO-1 Expression in Macrophages. International Journal of Molecular Sciences, 15(5), 8443-8457. https://doi.org/10.3390/ijms15058443