Effect of a Novel Quaternary Ammonium Methacrylate Polymer (QAMP) on Adhesion and Antibacterial Properties of Dental Adhesives
Abstract
:1. Introduction
2. Results
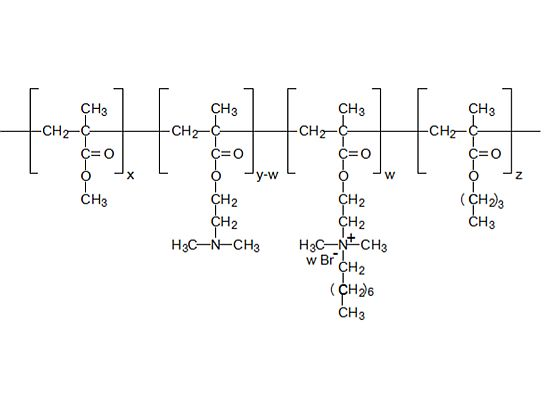
2.1. Micro-Raman Spectroscopy of Quaternary Ammonium Methacrylate Polymer (QAMP)
2.2. Resin–Dentin Bond Strength (μTBS)
2.3. In Situ Analysis of Degree of Conversion (DC)
2.4. In Vitro Analysis of Degree of Conversion (DC)
2.5. In Vitro Antibacterial Evaluation
3. Discussion
4. Materials and Methods
4.1. Micro-Raman Spectroscopy of the Quaternary Ammonium Methacrylate Polymer (QAMP)
4.2. Experimental Groups and Restorative Procedures
4.3. Teeth Selection and Preparation
4.4. Resin–Dentin Bond Strength (μTBS)
4.5. In Situ Analysis of Degree of Conversion (DC)
4.6. In Vitro Analysis of Degree of Conversion (DC)
4.7. In Vitro Antibacterial Evaluation
5. Conclusions
Conflicts of Interest
References
- Feuerstein, O.; Matalon, S.; Slutzky, H.; Weiss, E.I. Antibacterial properties of self-etching dental adhesive systems. J. Am. Dent. Assoc 2007, 138, 349–354. [Google Scholar]
- Walter, R.; Duarte, W.R.; Pereira, P.N.R.; Heymann, H.O.; Swift, E.J., Jr.; Arnold, R.R. In vitro inhibition of bacterial growth using different dental adhesive systems. Oper. Dent 2007, 32, 388–393. [Google Scholar]
- Imazato, S.; Imai, T.; Russell, R.R.; Torii, M.; Ebisu, S. Antibacterial activity of cured dental resin incorporating the antibacterial monomer MDPB and an adhesion-promoting monomer. J. Biomed. Mater. Res 1998, 39, 511–515. [Google Scholar]
- Bapna, M.S.; Murphy, R.; Mukherjee, S. Inhibition of bacterial colonization by antibacterial agents incorporated into dental resins. J. Oral Rehabil 1988, 15, 405–411. [Google Scholar]
- Kudou, Y.; Obara, K.; Kawashima, T.; Kubota, M.; Abe, S.; Endo, T.; Komatsu, M.; Okuda, R. Addition of antibacterial agents to MMA-TBB dentin bonding systems-influence on tensile bond strength and antibacterial effect. Dent. Mater. J 2000, 19, 65–74. [Google Scholar]
- Imazato, S.; Kuramoto, A.; Kaneko, T.; Ebisu, S.; Russell, R.R. Comparison of antibacterial activity of simplified adhesive systems. Am. J. Dent 2002, 15, 356–360. [Google Scholar]
- Kazuno, T.; Fukushima, T.; Hayakawa, T.; Inoue, Y.; Ogura, R.; Kaminishi, H.; Miyazaki, K. Antibacterial activities and bonding of MMSA/TBB resin containing amphiphilic lipids. Dent. Mater. J 2005, 24, 244–250. [Google Scholar]
- Fang, M.; Chen, J.H.; Xu, X.L.; Yang, P.H.; Hildebrand, H.F. Antibacterial activities of inorganic agents on six bacteria associated with oral infections by two susceptibility tests. Int. J. Antimicrob. Agents 2006, 27, 513–517. [Google Scholar]
- Fang, M.; Chai, F.; Chen, J.H.; Neut, C.; Jia, M.; Liu, Y.; Zhao, S.J.; Hildebrand, H.F. Antibacterial functionalization of an experimental self-etching primer by inorganic agents: Microbiological and biocompatibility evaluations. Biomol. Eng 2007, 24, 483–488. [Google Scholar]
- Meiers, J.C.; Shook, L.W. Effect of disinfectants on the bond strength of composite to dentin. Am. J. Dent 1996, 9, 11–14. [Google Scholar]
- Moen, B.; Rudi, K.; Bore, E.; Langsrud, S. Subminimal inhibitory concentrations of the disinfectant benzalkonium chloride select for a tolerant subpopulation of Escherichia coli with inheritable characteristics. Int. J. Mol. Sci 2012, 13, 4101–4123. [Google Scholar]
- Gürgan, S.; Bolay, S.; Kiremitçi, A. Effect of disinfectant application methods on the bond strength of composite to dentin. J. Oral Rehabil 1999, 26, 836–840. [Google Scholar]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Ebisu, S.; Tay, F.R. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dent. Mater 2003, 19, 313–319. [Google Scholar]
- Imazato, S.; Kaneko, T.; Takahashi, Y.; Noiri, Y.; Ebisu, S. In vivo antibacterial effects of dentin primer incorporating MDPB. Oper. Dent 2004, 29, 369–375. [Google Scholar]
- Imazato, S.; Tay, F.R.; Kaneshiro, A.V.; Takahashi, Y.; Ebisu, S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent. Mater 2007, 23, 170–176. [Google Scholar]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J 2009, 28, 11–19. [Google Scholar]
- Li, F.; Chai, Z.G.; Sun, M.N.; Wang, F.; Ma, S.; Zhang, L.; Fang, M.; Chen, J.H. Anti-biofilm effect of dental adhesive with cationic monomer. J. Dent. Res 2009, 88, 372–376. [Google Scholar]
- Xiao, Y.H.; Ma, S.; Chen, J.H.; Chai, Z.G.; Li, F.; Wang, Y.J. Antibacterial activity and bonding ability of an adhesive incorporating an antibacterial monomer DMAE-CB. J. Biomed. Mater. Res. B 2009, 90, 813–817. [Google Scholar]
- Thomé, T.; Mayer, M.P.; Imazato, S.; Geraldo-Martins, V.R.; Marques, M.M. In vitro analysis of inhibitory effects of the antibacterial monomer MDPB-containing restorations on the progression of secondary root caries. J. Dent 2009, 37, 705–711. [Google Scholar]
- Imazato, S.; Kuramoto, A.; Takahashi, Y.; Ebisu, S.; Peters, M.C. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent. Mater 2006, 22, 527–532. [Google Scholar]
- Imazato, S.; Kawakami, M.; Torii, M.; Tsuchitani, Y. Antibacterial activity of composites containing chemically bound non-releasing antibacterial component. J. Dent. Res 1992, 72, 721. [Google Scholar]
- Imazato, S.; Torii, M.; Tsuchitani, Y.; McCabe, J.F.; Russell, R.R.B. Incorporation of bacterial inhibitor into resin composite. J. Dent. Res 1994, 73, 1437–1443. [Google Scholar]
- Imazato, S.; Russell, R.R.B.; McCabet, J.F. Antibacterial activity of MDPB polymer incorporated in dental resin. J. Dent 1995, 23, 177–181. [Google Scholar]
- Imazato, S.; Ebi, N.; Tarumi, H.; Russell, R.R.B.; Kaneko, T.; Ebisu, S. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials 1999, 20, 899–903. [Google Scholar]
- Lu, G.; Wu, D.; Fu, R. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React. Funct. Polym 2007, 67, 355–366. [Google Scholar]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic antimicrobial polymers and their assemblies. Int. J. Mol. Sci 2013, 14, 9906–9946. [Google Scholar]
- Pupo, Y.M.; Farago, P.V.; Nadal, J.M.; Esmerino, L.A.; Maluf, D.F.; Zawadzki, S.F.; Michél, M.D.; Santos, F.A.; Gomes, O.M.M.; Gomes, J.C. An innovative quaternary ammonium methacrylate polymer can provide improved antibacterial properties for a dental adhesive system. J. Biomater. Sci. Polym. Ed 2013, 24, 1443–1458. [Google Scholar]
- Farago, P.V.; Pupo, Y.M.; Gomes, J.C. Processo de obtenção e uso de polímero metacrílico quaternário de amônio com propriedades antimicrobianas para a incorporação em materiais odontológicos. Brazil patent application number 10 2012 0266 4 18 December 2012. [Google Scholar]
- Poitevin, A.; de Munck, J.; van Landuyt, K.; Coutinho, E.; Peumans, M.; Lambrechts, P.; van Meerbeek, B. Critical analysis of the influence of different parameters on microtensile bond strength of adhesives to dentin. J. Adhes. Dent 2008, 10, 7–16. [Google Scholar]
- De Munck, J.; van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res 2005, 84, 118–132. [Google Scholar]
- Rullmann, I.; Schattenberg, A.; Marx, M.; Willershausen, B.; Ernst, C.P. Photoelastic determination of polymerization shrinkage stress in low-shrinkage resin composites. Schweiz. Monatsschr. Zahnmed 2012, 122, 294–299. [Google Scholar]
- Yamazaki, P.C.V.; Bedran-Russo, A.K.B.; Pereira, P.N.R.; Swift, E.J., Jr. Microleakage evaluation of a new low-shrinkage composite restorative material. Oper. Dent 2006, 31, 670–676. [Google Scholar]
- Pashley, D.H.; Carvalho, R.M.; Sano, H.; Nakajima, M.; Yoshiyama, M.; Shono, Y.; Fernandes, C.A.; Tay, F. The microtensile bond test: A review. J. Adhes. Dent 1999, 1, 299–309. [Google Scholar]
- Miyazaki, M.; Onose, H.; Moore, B.K. Analysis of the dentin-resin interface by use of laser Raman spectroscopy. Dent. Mater 2002, 18, 576–580. [Google Scholar]
- Navarra, C.O.; Cadenaro, M.; Armstrong, S.R.; Jessop, J.; Antoniolli, F.; Sergo, V.; di Lenarda, R.D.; Breschi, L. Degree of conversion of Filtek Silorane Adhesive System and Clearfil SE Bond within the hybrid and adhesive layer: An in situ Raman analysis. Dent. Mater 2009, 25, 1178–1185. [Google Scholar]
- Karamoddini, M.K.; Fazli-Bazzaz, B.S.; Emamipour, F.; Ghannad, M.S.; Jahanshahi, A.R.; Saed, N.; Sahebkar, A. Antibacterial efficacy of lytic bacteriophages against antibiotic-resistant Klebsiella species. Sci. World J 2011, 11, 1332–1340. [Google Scholar]
- Veij, M.; Vandenabeele, P.; Beer, T.; Remonc, J.P.; Moensa, L. Reference database of Raman spectra of pharmaceutical excipients. J. Raman Spectrosc 2009, 40, 297–307. [Google Scholar]
- Pigorsch, E. Spectroscopic characterization of cationic quaternary ammonium starches. Starch-Stärke 2009, 61, 129–138. [Google Scholar]
- Carvalho, R.M.; Manso, A.P.; Geraldeli, S.; Tay, F.R.; Pashley, D.H. Durability of bonds and clinical success of adhesive restorations. Dent. Mater 2012, 28, 72–86. [Google Scholar]
- Van Landuyt, K.L.; Snauwaert, J.; de Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar]
- Hass, V.; Luque-Martinez, I.; Sabino, N.B.; Loguercio, A.D.; Reis, A. Prolonged exposure times of one-step self-etch adhesives on adhesive properties and durability of dentine bonds. J. Dent 2012, 40, 1090–1102. [Google Scholar]
- Marchesi, G.; Navarra, C.O.; Cadenaro, M.; Carrilho, M.R.; Codan, B.; Sergo, V.; di Lenarda, R.; Breschi, L. The effect of ageing on the elastic modulus and degree of conversion of two multistep adhesive systems. Eur. J. Oral Sci 2010, 118, 304–310. [Google Scholar]
- Arrais, C.A.; Pontes, F.M.; Santos, L.P.; Leite, E.R.; Giannini, M. Degree of conversion of adhesive systems light-cured by LED and halogen light. Braz. Dent. J 2007, 18, 54–59. [Google Scholar]
- Tay, F.R.; Pashley, D.H. Aggressiveness of contemporary self-etching systems. I: Depth of penetration beyond dentin smear layers. Dent. Mater 2001, 17, 296–308. [Google Scholar]
- Chee, B.; Rickman, L.J.; Satterthwaite, J.D. Adhesives for the restoration of non-carious cervical lesions: A systematic review. J. Dent 2012, 40, 443–452. [Google Scholar]
- Kitasako, Y.; Burrow, M.F.; Nikaido, T.; Tagami, J. The influence of storage on dentin bond durability of resin cement. Dent. Mater 2000, 16, 1–6. [Google Scholar]
- Xiao, Y.H.; Chen, J.H.; Fang, M.; Xing, X.D.; Wang, H.; Wang, Y.J.; Li, F. Antibacterial effects of three experimental quaternary ammonium salts (QAS) monomers on bacteria associated with oral infections. J. Oral Sci 2008, 50, 323–327. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI), Performance standards for antibacterial susceptibility testing; Twenty-first informational supplement. In CLSI Document M100-S21; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011.




| Adhesive system | Storage period | ||
|---|---|---|---|
| Immediately | 6 months | 12 months | |
| Clearfil™ SE Bond containing 5% QAMP | 34.88 ± 8.71 Aa | 33.43 ± 5.47 Aa | 32.50 ± 5.94 Aa |
| Clearfil™ Protect Bond | 37.23 ± 6.71 Aa | 39.44 ± 3.25 Aa | 28.20 ± 8.09 Ba |
| Clearfil™ SE Bond | 34.69 ± 5.95 Aa | 32.08 ± 10.97 Aa | 31.27 ± 9.30 Aa |
| Adhesive system | Storage period | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immediately | 6 months | 12 months | ||||||||||
| A/M | C | Debond | T | A/M | C | Debond | T | A/M | C | Debond | T | |
| Clearfil™ SE Bond containing 5% QAMP | 36(80) | 0(0) | 9(20) | 45 | 37(92.5) | 2(5) | 1(2.5) | 40 | 34(94.5) | 0(0) | 2(5.5) | 36 |
| Clearfil™ Protect Bond | 35(79.6) | 0(0) | 9(20.4) | 44 | 35(83.3) | 1(2.4) | 6(14.3) | 42 | 34(91.9) | 1(2.5) | 3(8.1) | 37 |
| Clearfil™ SE Bond | 35(71.4) | 0(0) | 14(28.6) | 49 | 35(81.4) | 0(0) | 8(18.6) | 43 | 35(83.3) | 0(0) | 7(16.7) | 42 |
| Adhesive systems | ||
|---|---|---|
| Clearfil™ SE Bond containing 5% QAMP | Clearfil™ Protect Bond | Clearfil™ SE Bond |
| 89 ± 6 a | 85 ± 11 a | 88 ± 3 a |
| Adhesive systems | ||
|---|---|---|
| Clearfil™ SE Bond containing 5% QAMP | Clearfil™ Protect Bond | Clearfil™ SE Bond |
| 39.7 ± 2.9 a | 39.8 ± 4.9 a | 40.0 ± 3.0 a |
| Adhesive system | MIC/MBC values (μL/mL) | ||
|---|---|---|---|
| Streptococcus mutans (ATCC 25175) | Lactobacillus casei (ATCC 393) | Actinomyces naesludii (ATCC 12104) | |
| Clearfil™ SE Bond containing 5% QAMP | 20/20 | 10/20 | 20/20 |
| Clearfil™ Protect Bond | 10/10 | 10/20 | 20/20 |
| Clearfil™ SE Bond | 80/80 | 80/>80 | 80/80 |
| Adhesive systems | Composition (batch number) | Application mode |
|---|---|---|
| Clearfil™ SE Bond containing 5% QAMP | Primer: MDP, HEMA, hydrophilic dimethacrylate, di-camphorquinone, N,N-diethanol-p-toluidine, water, QAMP. (00954A) Bond: MDP, Bis-GMA, HEMA, hydrophobic dimethacrylate, di-camphorquinone, N,N-diethanol-p-toluidine, silanated colloidal silica. (01415A) | Application of two coats of the primer under pressure (20 s); Gentle air stream (10 s at 20 cm) after application of each coat; Application of one coat of the adhesive (15 s); Gentle air stream to make the bond film uniform (3 s at 20 cm); Light cured (10 s at 600 mW/cm2). |
| Clearfil™ Protect Bond | Primer: MDPB, MDP, HEMA, hydrophilic dimethacrylate, water. (00081B) Bond: MDP, HEMA, Bis-GMA, hydrophobic dimethacrylate, di-camphorquinone, N,N-diethanol-p-toluidine, silanated colloidal silica, surface-treated sodium fluoride. (00134B) | |
| Clearfil™ SE Bond | Primer: MDP, HEMA, hydrophilic dimethacrylate, di-camphorquinone, N,N-diethanol-p-toluidine, water. (00954A) Bond: MDP, Bis-GMA, HEMA, hydrophobic dimethacrylate, di-camphorquinone, N,N-diethanol-p-toluidine, silanated colloidal silica. (01415A) |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pupo, Y.M.; Farago, P.V.; Nadal, J.M.; Simão, L.C.; Esmerino, L.A.; Gomes, O.M.M.; Gomes, J.C. Effect of a Novel Quaternary Ammonium Methacrylate Polymer (QAMP) on Adhesion and Antibacterial Properties of Dental Adhesives. Int. J. Mol. Sci. 2014, 15, 8998-9015. https://doi.org/10.3390/ijms15058998
Pupo YM, Farago PV, Nadal JM, Simão LC, Esmerino LA, Gomes OMM, Gomes JC. Effect of a Novel Quaternary Ammonium Methacrylate Polymer (QAMP) on Adhesion and Antibacterial Properties of Dental Adhesives. International Journal of Molecular Sciences. 2014; 15(5):8998-9015. https://doi.org/10.3390/ijms15058998
Chicago/Turabian StylePupo, Yasmine M., Paulo Vitor Farago, Jessica M. Nadal, Luzia C. Simão, Luís Antônio Esmerino, Osnara M. M. Gomes, and João Carlos Gomes. 2014. "Effect of a Novel Quaternary Ammonium Methacrylate Polymer (QAMP) on Adhesion and Antibacterial Properties of Dental Adhesives" International Journal of Molecular Sciences 15, no. 5: 8998-9015. https://doi.org/10.3390/ijms15058998
APA StylePupo, Y. M., Farago, P. V., Nadal, J. M., Simão, L. C., Esmerino, L. A., Gomes, O. M. M., & Gomes, J. C. (2014). Effect of a Novel Quaternary Ammonium Methacrylate Polymer (QAMP) on Adhesion and Antibacterial Properties of Dental Adhesives. International Journal of Molecular Sciences, 15(5), 8998-9015. https://doi.org/10.3390/ijms15058998