The Functions of BMP3 in Rabbit Articular Cartilage Repair
Abstract
:1. Introduction
2. Results
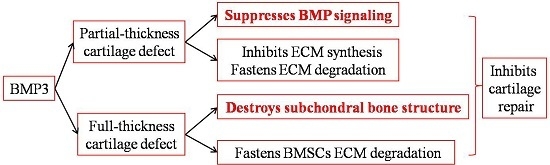
2.1. The Effect of BMP3 on Partial-Thickness Defects

2.2. The Effect of BMP3 on Full-Thickness Defect

2.3. The Effect of BMP3 on Chondrocyte Proliferation and ECM Synthesis


2.4. The Effect of BMP3 on BMSCs Proliferation and Differentiation


3. Discussion

4. Experimental Section
4.1. Preparation of BMP3-Loaded Collagen and Collagen (Col)/Hydroxyapatite (Hap) Membranes
4.2. Animal Model
4.3. Macroscopic and Histological Evaluation
4.4. Immunohistochemistry for Collagen Type II
4.5. Isolation of Articular Chondrocytes and BMSCs
4.6. Immunofluorescence Staining of Collagen Type II
4.7. RNA Extraction
4.8. Reverse Transcription Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
| Gene | Reference Sequence | Primer Sequence (5′ to 3′) | Product Size (bp) |
|---|---|---|---|
| GAPDH | NM001082253 | F: GCC CCT CTT CAC AGT TTC CA | 97 |
| R: GCT GTC GAG ACT TTA TTG ATG GT | |||
| SOX9 | XM008271763.1 | F: CAA GAA AGA CCA CCC GGA CT | 123 |
| R: GCC TTG AAG ATG GCG TTG GG | |||
| COL1A2 | NM001195668.1 | F: CCA TCT CGT TTG CCC TTC CT | 80 |
| R: GGG CCA ACG TCC ACA TAG AA | |||
| COL2A1 | NM001195671 | F: TGC AGG AGG GGA AGA GGT AT | 123 |
| R: GGC AGT CCT TGG TGT CTT CA | |||
| BMP2 | NM001082650 | F: CGC CTC AAA TCC AGC TGT AAG | 80 |
| R: GGG CCA CAA TCC AGT CGT T | |||
| BMP4 | NM001195723 | F: ACC GAA TGC TGA TGG TCG TT | 84 |
| R: TCT TCC CCG TCT CAG GTA TCA | |||
| MMP13 | NM001082037 | F: CTG ACT AGG AAG CGG AAG CC | 125 |
| R: ACA CCT GGC TGC ATC TTG AA |
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Mastbergen, S.C.; Saris, D.B.; Lafeber, F.P. Functional articular cartilage repair: Here, near, or is the best approach not yet clear? Nat. Rev. Rheumatol. 2013, 9, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Madeira, C.; Santhagunam, A.; Salgueiro, J.B.; Cabral, J.M.S. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol. 2015, 33, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Pulsatelli, L.; Facchini, A. Signaling pathways in cartilage repair. Int. J. Mol. Sci. 2014, 15, 8667–8698. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Mrugala, D.; Jorgensen, C.; Guicheux, J.; Noel, D. Cartilage engineering: A crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009, 27, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.C.; Bertrand, J.; Eldridge, S.E.; Dell’Accio, F. Cellular and molecular mechanisms of cartilage damage and repair. Drug Discov. Today 2014, 19, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Rosen, V. BMP and BMP inhibitors in bone. Annal. N. Y. Acad. Sci. 2006, 1068, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, G.; Wang, J.; Zhou, Z.; Li, L.; Liu, L.; Guan, M.; Zhang, Q.; Wang, H. Exogenous bFGF promotes articular cartilage repair via up-regulation of multiple growth factors. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2013, 21, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Luyten, F.P.; Cunningham, N.S.; Ma, S.; Muthukumaran, N.; Hammonds, R.G.; Nevins, W.B.; Woods, W.; Reddi, A. Purification and partial amino acid sequence of osteogenin, a protein initiating bone differentiation. J. Biol. Chem. 1989, 264, 13377–13380. [Google Scholar] [PubMed]
- Bahamonde, M.E.; Lyons, K.M. BMP3: To be or not to be a BMP. J. Bone Jt. Surg. 2001, 83, S56–S62. [Google Scholar]
- Daluiski, A.; Engstrand, T.; Bahamonde, M.E.; Gamer, L.W.; Agius, E.; Stevenson, S.L.; Cox, K.; Rosen, V.; Lyons, K.M. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat. Genet. 2001, 27, 84–88. [Google Scholar] [PubMed]
- Gamer, L.W.; Nove, J.; Levin, M.; Rosen, V. BMP-3 is a novel inhibitor of both activin and BMP-4 signaling in xenopus embryos. Dev. Biol. 2005, 285, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Gamer, L.W.; Ho, V.; Cox, K.; Rosen, V. Expression and function of BMP3 during chick limb development. Dev. Dyn. 2008, 237, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Gamer, L.W.; Cox, K.; Carlo, J.M.; Rosen, V. Overexpression of BMP3 in the developing skeleton alters endochondral bone formation resulting in spontaneous rib fractures. Dev. Dyn. 2009, 238, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Ito-Amano, M.; Nakamura, Y.; Morisaki, M.; He, X.; Hayashi, M.; Watanapokasin, R.; Kato, H. Temporal and spatial expression patterns of bone morphogenetic protein 3 in developing zebrafish. Open Rheumatol. J. 2014, 8, 69–72. [Google Scholar] [PubMed]
- Zhang, W.; Chen, J.; Tao, J.; Jiang, Y.; Hu, C.; Huang, L.; Ji, J.; Ouyang, H.W. The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair. Biomaterials 2013, 34, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Rosenberg, L.C. Repair of partial-thickness defects in articular cartilage: Cell recruitment from the synovial membrane. J. Bone Jt. Surg. Am. 1996, 78, 721–733. [Google Scholar]
- Barry, F.; Murphy, M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 2013, 9, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, B.; Yang, J.; Xin, L.; Li, Y.; Yin, H.; Qi, Y.; Jiang, Y.; Ouyang, H.; Gao, C. The restoration of full-thickness cartilage defects with BMSCs and TGF-β 1 loaded PLGA/fibrin gel constructs. Biomaterials 2010, 31, 8964–8973. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F.; Koide, S.; Glimcher, M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J. Bone Jt. Surg. Am. 1993, 75, 532–553. [Google Scholar]
- Murphy, M.K.; Huey, D.J.; Hu, J.C.; Athanasiou, K.A. TGF-β1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells 2015, 33, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Lópiz-Morales, Y.; Abarrategi, A.; Ramos, V.; Moreno-Vicente, C.; López-Durán, L.; López-Lacomba, J.L.; Marco, F. In vivo comparison of the effects of rhBMP-2 and rhBMP-4 in osteochondral tissue regeneration. Eur. Cells Mater. 2010, 20, 367–378. [Google Scholar]
- Stewart, A.; Guan, H.; Yang, K. BMP-3 promotes mesenchymal stem cell proliferation through the TGF-β/activin signaling pathway. J. Cell. Physiol. 2010, 223, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, S.; Luyten, F.P.; Reddi, A. Stimulation of the expression of osteogenic and chondrogenic phenotypes in vitro by osteogenin. Proc. Natl. Acad. Sci. USA 1989, 86, 8793–8797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tao, Y.; Liang, C.; Zhang, Y.; Li, H.; Chen, Q. BMP3 alone and together with TGF-β promote the differentiation of human mesenchymal stem cells into a nucleus pulposus-like phenotype. Int. J. Mol. Sci. 2015, 16, 20344–20359. [Google Scholar] [CrossRef] [PubMed]
- Kloen, P.; Lauzier, D.; Hamdy, R.C. Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone 2012, 51, 59–68. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.S. Bone morphogenic protein 3 signaling in the regulation of osteogenesis. Orthopedics 2012, 35, 920. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-D.; Jin, W.; Hao, X.-D.; Tang, N.L.S.; Zhang, Y.-P. Evidence for positive selection on the osteogenin (BMP3) gene in human populations. PLoS ONE 2010, 5, e10959. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yamashiro, T.; Fukunaga, T.; Balam, T.A.; Takano-Yamamoto, T. Bone morphogenetic protein 3 expression pattern in rat condylar cartilage, femoral cartilage and mandibular fracture callus. Eur. J. Oral Sci. 2005, 113, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, Q.; Liang, Q.Q.; Li, C.G.; Holz, J.D.; Tang, D.; Sheu, T.J.; Li, T.F.; Shi, Q.; Wang, Y.J. IGF-1 regulation of type ii collagen and MMP-13 expression in rat endplate chondrocytes via distinct signaling pathways. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2009, 17, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, W.; Li, F.; Guo, F.; Chen, A. Bortezomib prevents the expression of MMP-13 and the degradation of collagen type 2 in human chondrocytes. Biochem. Biophys. Res. Commun. 2014, 452, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Kokabu, S.; Gamer, L.; Cox, K.; Lowery, J.; Tsuji, K.; Raz, R.; Economides, A.; Katagiri, T.; Rosen, V. BMP3 suppresses osteoblast differentiation of bone marrow stromal cells via interaction with Acvr2b. Mol. Endocrinol. 2012, 26, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 is required for cartilage formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [PubMed]
- Myllyharju, J.; Kivirikko, K.I. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004, 20, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Borzí, R.M.; Olivotto, E.; Pagani, S.; Vitellozzi, R.; Neri, S.; Battistelli, M.; Falcieri, E.; Facchini, A.; Flamigni, F.; Penzo, M.; et al. Matrix metalloproteinase 13 loss associated with impaired extracellular matrix remodeling disrupts chondrocyte differentiation by concerted effects on multiple regulatory factors. Arthritis Rheum. 2010, 62, 2370–2381. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Chen, D. The bmp signaling and in vivo bone formation. Gene 2005, 357, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, L.; Ren, L.; Wang, F. Preparation and characterization of collagen-chitosan composites. J. Appl. Polym. Sci. 1997, 64, 2127–2130. [Google Scholar] [CrossRef]
- Ding, Q.; Zhong, H.; Qi, Y.; Cheng, Y.; Li, W.; Yan, S.; Wang, X. Anti-arthritic effects of crocin in interleukin-1β-treated articular chondrocytes and cartilage in a rabbit osteoarthritic model. Inflamm. Res. 2013, 62, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.W.; Goh, J.C.; Thambyah, A.; Teoh, S.H.; Lee, E.H. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit achilles tendon. Tissue Eng. 2003, 9, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-Y.; Chen, C.-H.; Hsiao, C.-Y.; Chen, J.-P. Incorporation of chitosan in biomimetic gelatin/chondroitin-6-sulfate/hyaluronan cryogel for cartilage tissue engineering. Carbohydr. Polym. 2015, 117, 722–730. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yang, W.; Cao, Y.; Shi, Y.; Lei, C.; Du, B.; Li, X.; Zhang, Q. The Functions of BMP3 in Rabbit Articular Cartilage Repair. Int. J. Mol. Sci. 2015, 16, 25934-25946. https://doi.org/10.3390/ijms161125937
Zhang Z, Yang W, Cao Y, Shi Y, Lei C, Du B, Li X, Zhang Q. The Functions of BMP3 in Rabbit Articular Cartilage Repair. International Journal of Molecular Sciences. 2015; 16(11):25934-25946. https://doi.org/10.3390/ijms161125937
Chicago/Turabian StyleZhang, Zhe, Wenyu Yang, Yiting Cao, Yanping Shi, Chen Lei, Bo Du, Xuemin Li, and Qiqing Zhang. 2015. "The Functions of BMP3 in Rabbit Articular Cartilage Repair" International Journal of Molecular Sciences 16, no. 11: 25934-25946. https://doi.org/10.3390/ijms161125937
APA StyleZhang, Z., Yang, W., Cao, Y., Shi, Y., Lei, C., Du, B., Li, X., & Zhang, Q. (2015). The Functions of BMP3 in Rabbit Articular Cartilage Repair. International Journal of Molecular Sciences, 16(11), 25934-25946. https://doi.org/10.3390/ijms161125937