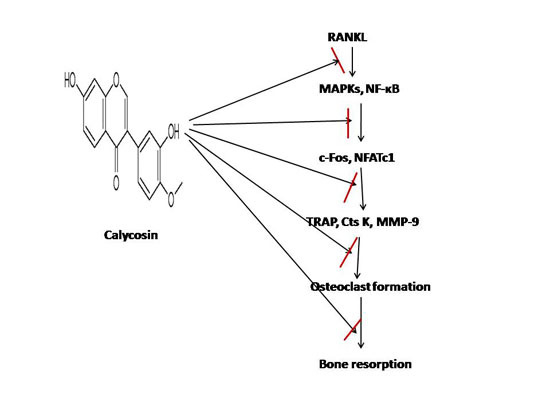
Calycosin Suppresses RANKL-Mediated Osteoclastogenesis through Inhibition of MAPKs and NF-κB
Abstract
:1. Introduction
2. Results
2.1. Calycosin Inhibits RANKL-Induced Osteoclast Formation


2.2. Calycosin Reduces RANKL-Induced Bone Resorption by Osteoclasts


2.3. Calycosin Inhibits RANKL-Induced Expression of Osteoclastic Marker Genes

2.4. Calycosin Suppresses RANKL-Induced c-Fos and NFATc1 Expression

2.5. Calycosin Inhibits RANKL-Induced NF-κB Activation
2.6. Calycosin Inhibits RANKL-Induced MAPK Phosphorylation



3. Discussion
4. Experimental Section
4.1. Reagents
4.2. Cell Culture
4.3. Assessment of Cell Viability
4.4. Osteoclast Differentiation and TRAP Staining Assay
4.5. Bone Resorption Assay and Modified Von Kossa Staining
4.6. RNA Extraction and Real-Time Quantitative RT-PCR
| Primers | Gene Sequence |
|---|---|
| mouse CtsK forward | 5′-AATACCTCCCTCTCGATCCTACA-3′ |
| mouse CtsK reverse | 5′-TGGTTCTTGACTGGAGTAACGTA-3′ |
| mouse MMP-9 forward | 5′-CTGGACAGCCAGACACTAAAG-3′ |
| mouse MMP-9 reverse | 5′-CTCGCGGCAAGTCTTCAGAG-3′ |
| mouse TRAP forward | 5′-CACTCCCACCCTGAGATTTGT-3′ |
| mouse TRAP reverse | 5′-CATCGTCTGCACGGTTCTG-3′ |
| mouse NFATc1 forward | 5′-GGAGAGTCCGAGAATCGAGAT-3′ |
| mouse NFATc1 reverse | 5′-TTGCAGCTAGGAAGTACGTCT-3′ |
| mouse c-Fos forward | 5′-CGGGTTTCAACGCCGACTA-3′ |
| mouse c-Fos reverse | 5′-TTGGCACTAGAGACGGACAGA-3′ |
| mouse β-actin forward | 5′-GGCTGTATTCCCCTCCATCG-3′ |
| mouse β-actin reverse | 5′-CCAGTTGGTAACAATGCCATGT-3′ |
4.7. Western Blotting
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rho, J.; Takami, M.; Choi, Y. Osteoimmunology: Interactions of the immune and skeletal systems. Mol. Cell 2004, 17, 1–9. [Google Scholar]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, M.; Giro, G.; Taubman, M.A.; Han, X.; Mayer, M.P.; Kawai, T. Role of periodontal pathogenic bacteriain RANKL-mediated bone destruction in periodontal disease. J. Oral Microbiol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Dass, C.R.; Choong, P.F. Novel therapeutic strategy for osteosarcoma targeting osteoclast differentiation, bone-resorbing activity, and apoptosis pathway. Mol. Cancer Ther. 2008, 7, 3461–3469. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H.J.; Lee, D.; Lee, T.; Shin, H.I.; Bae, Y.C.; Kim, S.H.; Park, E.K. The 1,2,3-triazole derivative KP-A021 suppresses osteoclast differentiation and function by inhibiting RANKL-mediated MEK-ERK signaling pathway. Exp. Biol. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoclast differentiation and activation. Clin. Calcium 2007, 17, 484–492. [Google Scholar] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Logar, D.B.; Komadina, R.; Prezelj, J.; Ostanek, B.; Trost, Z.; Marc, J. Expression of bone resorption genes in osteoarthritis and in osteoporosis. J. Bone Miner. Metab. 2007, 25, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Xie, X.; Zhu, Z.; Yu, X.; Liu, H.; Wang, H.; Fan, H.; Wang, D.; Jiang, G.; Hong, M. Screening active components from Yu-ping-feng-san for regulating initiative key factors in allergic sensitization. PLoS ONE 2014, 9, e107279. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Feng, L.; Wang, S.; Zhu, Q.; Zheng, Z.; Xiang, P.; He, B.; Tang, D. Calycosin protects HUVECs from advanced glycation end products-induced macrophage infiltration. J. Ethnopharmacol. 2011, 137, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, X.; Li, X.; Wu, Y. Calycosin induces apoptosis by the regulation of ERβ/miR-17 signaling pathway in human colorectal cancer cells. Food Funct. 2015, 6, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Q.H.; Liu, C.L.; Lin, L. Calycosin induces apoptosis in human ovarian cancer SKOV3 cells by activating caspases and Bcl-2 family proteins. Tumour Biol. 2015, 36, 5333–5339. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Ma, G.; Zheng, C.; Qiu, X.; Li, X.; Mo, J.; Li, Z.; Liu, Y.; Mo, L.; Bi, G.; Ye, Y. Antineoplastic effect of calycosin on osteosarcoma through inducing apoptosis showing in vitro and in vivo investigations. Exp. Mol. Pathol. 2014, 97, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.G.; Li, N.; Lau, K.M.; Lee, P.S.; Yan, L.; Xu, M.L.; Lam, C.T.; Kong, A.Y.; Lin, H.Q.; Dong, T.T.; et al. Calycosin orchestrates the functions of DangguiBuxue Tang, a Chinese herbal decoction composing of Astragali Radix and Angelica Sinensis Radix: An evaluation by using calycosin-knock out herbal extract. J. Ethnopharmacol. 2015, 168, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, F.; Nishimura, R.; Matsubara, T.; Tanaka, S.; Inoue, J.; Reddy, S.V.; Hata, K.; Yamashita, K.; Hiraga, T.; Watanabe, T.; et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J. Clin. Investig. 2004, 114, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kogawa, M.; Wada, S.; Takayanagi, H.; Tsujimoto, M.; Katayama, S.; Hisatake, K.; Nogi, Y. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J. Biol. Chem. 2004, 279, 45969–45979. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Sudo, T.; Maruyama, M.; Osada, H.; Tsujimoto, M. Activation of p38 mitogen-activated protein kinase is crucial in osteoclastogenesis induced by tumor necrosis factor. FEBS Lett. 2000, 486, 23–28. [Google Scholar] [CrossRef]
- Mediero, A.; Perez-Aso, M.; Cronstein, B.N. Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFkappaB nuclear translocation. Br. J. Pharmacol. 2013, 169, 1372–1388. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, J.K.; Jang, H.D. Genistein inhibits osteoclastic differentiation of RAW 264.7 cells via regulation of ROS production and scavenging. Int. J. Mol. Sci. 2014, 15, 10605–10621. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.E.; Lee, W.I.; Kang, J.W.; Nam, D.; Choi, D.Y.; Park, D.S.; Lee, S.H.; Lee, J.D. Formononetin attenuates osteoclastogenesis via suppressing the RANKL-induced activation of NF-κB, c-Fos, and nuclear factor of activated T-cells cytoplasmic 1 signaling pathway. J. Nat. Prod. 2014, 77, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Kang, L.; Ma, Y.; Chen, H.; Kuang, H.; Huang, Q.; He, M.; Peng, W. Effects and mechanisms of 8-prenylnaringenin on osteoblast MC3T3-E1 and osteoclast-like cells RAW264.7. Food Sci. Nutr. 2014, 2, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Karieb, S.; Fox, S.W. Phytoestrogens directly inhibit TNF-α-induced bone resorption in RAW264.7 cells by suppressing c-Fos-induced NFATc1 expression. J. Cell. Biochem. 2011, 112, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-κB ligand (RANKL) activation of NF-κB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Ovitt, C.; Grigoriadis, A.E.; Mohle-Steinlein, U.; Ruther, U.; Wagner, E.F. Bone and haematopoietic defects in mice lacking c-Fos. Nature 1992, 360, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Galson, D.L.; Zhao, C.; Peng, L.; Laplace, C.; Wang, K.Z.; Bachler, M.A.; Amano, H.; Aburatani, H.; Ishikawa, H.; et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 2004, 279, 26475–26480. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in Osteoclast Differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bohm, C.; Hayer, S.; Kilian, A.; Zaiss, M.M.; Finger, S.; Hess, A.; Engelke, K.; Kollias, G.; Kronke, G.; Zwerina, J.; et al. The α-isoform of p38 MAPK specifically regulates arthritic bone loss. J. Immunol. 2009, 183, 5938–5947. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Hirata, A.; Tsuji, T.; Yamamoto, T. Role of osteoclast extracellular signal-regulated kinase (ERK) in cell survival and maintenance of cell polarity. J. Bone Miner. Res. 2003, 18, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Cheon, Y.H.; Kwak, S.C.; Baek, J.M.; Kim, Y.C.; Yoon, K.H.; Oh, J.; Lee, M.S. 9-Hydroxy-6,7-dimethoxydalbergiquinol inhibits osteoclast differentiation through down-regulation of Akt, c-Fos and NFATc1. Int. Immunopharmacol. 2014, 20, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Aoki, K.; Saito, H.; D’Acquisto, F.; May, M.J.; Nakamura, I.; Sudo, T.; Kojima, T.; Okamoto, F.; Fukushima, H.; et al. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat. Med. 2004, 10, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, X.; Wang, H.B.; Wu, D.; Li, X.O.; Peng, Q.; Liu, N.; Sun, W.C. 17-Hydroxy-jolkinolide A inhibits osteoclast differentiation through suppressing the activation of NF-κB and MAPKs. Int. Immunopharmacol. 2015, 29, 513–520. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, G.-H.; Wang, H.; Cao, J.; Zhang, Y.; Wu, D.; Peng, Q.; Liu, N.; Sun, W.-C. Calycosin Suppresses RANKL-Mediated Osteoclastogenesis through Inhibition of MAPKs and NF-κB. Int. J. Mol. Sci. 2015, 16, 29496-29507. https://doi.org/10.3390/ijms161226179
Quan G-H, Wang H, Cao J, Zhang Y, Wu D, Peng Q, Liu N, Sun W-C. Calycosin Suppresses RANKL-Mediated Osteoclastogenesis through Inhibition of MAPKs and NF-κB. International Journal of Molecular Sciences. 2015; 16(12):29496-29507. https://doi.org/10.3390/ijms161226179
Chicago/Turabian StyleQuan, Gui-Hua, Hongbing Wang, Jinjin Cao, Yuxin Zhang, Donglin Wu, Qisheng Peng, Ning Liu, and Wan-Chun Sun. 2015. "Calycosin Suppresses RANKL-Mediated Osteoclastogenesis through Inhibition of MAPKs and NF-κB" International Journal of Molecular Sciences 16, no. 12: 29496-29507. https://doi.org/10.3390/ijms161226179
APA StyleQuan, G. -H., Wang, H., Cao, J., Zhang, Y., Wu, D., Peng, Q., Liu, N., & Sun, W. -C. (2015). Calycosin Suppresses RANKL-Mediated Osteoclastogenesis through Inhibition of MAPKs and NF-κB. International Journal of Molecular Sciences, 16(12), 29496-29507. https://doi.org/10.3390/ijms161226179