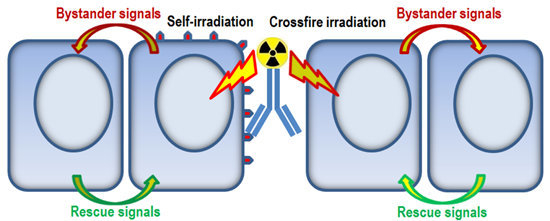
Rescue Effects: Irradiated Cells Helped by Unirradiated Bystander Cells
Abstract
:1. Introduction

2. Discovery of Rescue Effect
2.1. 53BP1 Foci in Irradiated Cells
2.2. MN Induction in Irradiated Cells
2.3. Apoptosis and Survival in Irradiated Cells
3. Other Studies Confirming the Rescue Effect
3.1. Rescue Effect in Human Melanoma (Me45) Cells and Human Dermal Fibroblasts (NHDF)
3.2. Rescue Effect in Embryonic Zebrafish Fibroblast (ZF4) Cells
3.3. Rescue Effect in Lung Adenocarcinoma (A549) Cells and Human Lung Normal Fibroblast (WI38) Cells


4. Mechanisms Underlying the Rescue Effect
4.1. Involvement of cAMP

4.2. NF-κB Activation


5. Rescue Effect between Irradiated and Bystander Zebrafish Embryos
6. Discussion
6.1. Rescue Effects Observed in in Vitro Experiments and Underlying Mechanisms
6.2. Rescue Effects Observed between Zebrafish Embryos
6.3. Rescue Effect in Radioimmunotherapy
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, S.; Zhao, Y.; Han, W.; Chiu, S.K.; Zhu, L.; Wu, L.; Yu, K.N. Rescue effects in radiobiology: Unirradiated bystander cells assist irradiated cells through intercellular signal feedback. Mutat. Res. 2011, 706, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, H.; Little, J.B. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992, 52, 6394–6396. [Google Scholar] [PubMed]
- Mothersill, C.; Seymour, C. Radiation induced bystander effects: Past history and future directions. Radiat. Res. 2001, 155, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Mothersill, C.; Seymour, C. Radiation induced bystander effects—Implications for cancer. Nat. Rev. 2004, 4, 158–164. [Google Scholar] [CrossRef]
- Goldberg, Z.; Lehnert, B.E. Radiation induced effects in unirradiated cells: A review and implications in cancer. Int. J. Oncol. 2002, 21, 337–349. [Google Scholar] [PubMed]
- Little, J.B. Cellular radiation effects and the bystander response. Mutat. Res. 2006, 597, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.F.; Sowa, M.B. Non-targeted bystander effects induced by ionizing radiation. Mutat. Res. 2007, 616, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Hei, T.K.; Zhou, H.; Ivanov, V.N.; Hong, M.; Lieberman, H.B.; Brenner, D.J.; Amundson, S.A.; Geard, C.R. Mechanism of radiation induced bystander effects: A unifying model. J. Pharm. Pharmacol. 2008, 60, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, K.N.; Hou, J.; Liu, Q.; Han, W. Radiation-induced bystander effect: Early process and rapid assessment. Cancer Lett. 2015, 356, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Shareef, M.M.; Cui, N.; Burikhanov, R.; Gupta, S.; Satishkumar, S.; Shajahan, S.; Mohiuddin, M.; Rangnekar, V.M.; Ahmed, M.M. Role of tumor necrosis factor-alpha and TRAIL in high-dose radiation-induced bystander signaling in lung adenocarcinoma. Cancer Res. 2007, 67, 11811–11820. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Lehnert, B.E.; Svensson, R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000, 60, 1290–1298. [Google Scholar] [PubMed]
- Chou, C.H.; Chen, P.J.; Lee, P.H.; Cheng, A.L.; Hsu, H.C.; Cheng, J.C. Radiation-induced hepatitis B virus reactivation in liver mediated by the bystander effect from irradiated endothelial cells. Clin. Cancer Res. 2007, 13, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Facoetti, A.; Ballarini, F.; Cherubini, R.; Gerardi, S.; Nano, R.; Ottolenghi, A.; Prise, K.M.; Trott, K.R.; Zilio, C. Gamma ray-induced bystander effect in tumour glioblastoma cells: A specific study on cell survival, cytokine release and cytokine receptors. Radiat. Prot. Dosim. 2006, 122, 271–274. [Google Scholar] [CrossRef]
- Han, W.; Wu, L.; Chen, S.; Bao, L.; Zhang, L.; Jiang, E.; Zhao, Y.; Xu, A.; Hei, T.K.; Yu, Z. Constitutive nitric oxide acting as a possible intercellular signaling molecule in the initiation of radiation-induced DNA double strand breaks in non-irradiated bystander cells. Oncogene 2007, 26, 2330–2339. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Folkard, M.; Prise, K.M. Role of TGF-β1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene 2008, 27, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hayashi, S.; Hatashita, M.; Ohnishi, K.; Shioura, H.; Ohtsubo, T.; Kitai, R.; Ohnishi, T.; Kano, E. Induction of radioresistance by a nitric oxide-mediated bystander effect. Radiat. Res. 2001, 155, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Furusawa, Y.; Kobayashi, Y.; Funayama, T.; Wada, S. Bystander effect induced by counted high-LET particles in confluent human fibroblasts: A mechanistic study. FASEB J. 2003, 17, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Widel, M.; Przybyszewski, W.M.; Cieslar-Pobuda, A.; Saenko, Y.V.; Rzeszowska-Wolny, J. Bystander normal human fibroblasts reduce damage response in radiation targeted cancer cells through intercellular ROS level modulation. Mutat. Res. 2012, 731, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Malard, V.; Ravanat, J.-L.; Davin, A.-H.; Armengaud, J.; Foray, N.; Adam-Guillermin, C. Low doses of gamma-irradiation induce an early bystander effect in zebrafish cells which is sufficient to radioprotect cells. PLoS One 2014, 9, e92974. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Kobayashi, A.; Konishi, T.; Oikawa, M.; Pandey, B.N. Damaging and protective bystander cross-talk between human lung cancer and normal cells after proton microbeam irradiation. Mutat. Res 2014, 763–764, 39–44. [Google Scholar] [CrossRef]
- He, M.; Dong, C.; Xie, Y.; Li, J.; Yuan, D.; Bai, Y.; Shao, C. Reciprocal bystander effect between α-irradiated macrophage and hepatocyte is mediated by cAMP through a membrane signaling pathway. Mutat. Res. 2014, 763–764, 1–9. [Google Scholar] [CrossRef]
- Lam, R.K.K.; Fung, Y.K.; Han, W.; Li, L.; Chiu, S.K.; Cheng, S.H.; Yu, K.N. Modulation of NF-κB in rescued irradiated cells. Radiat. Prot. Dosim. 2015, in press. [Google Scholar]
- Choi, V.W.Y.; Ng, C.Y.P.; Cheng, S.H.; Yu, K.N. α-Particle irradiated zebrafish embryos rescued by bystander unirradiated zebrafish embryos. Environ. Sci. Technol. 2012, 46, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.Y.; Choi, V.W.Y.; Cheng, S.H.; Yu, K.N. Some properties of the signals involved in unirradiated zebrafish embryos rescuing α-particle irradiated zebrafish embryos. Int. J. Radiat. Biol. 2014, 90, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Furusawa, Y.; Matsumoto, Y.; Pan, Y.; Xu, P.; Chen, H. Effect of gap junctional intercellular communication on radiation responses in neoplastic human cells. Radiat. Res. 2007, 167, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Naderi, E.H.; Jochemsen, A.G.; Blomhoff, H.K.; Naderi, S. Activation of cAMP signaling interferes with stress-induced p53 accumulation in ALL-derived cells by promoting the interaction between p53 and HDM2. Neoplasia 2011, 13, 653–663. [Google Scholar] [PubMed]
- Tergaonkar, V.; Pando, M.; Vafa, O.; Wahl, G.; Verma, I. p53 stabilization is decreased upon NFκB activation: A role for NFκB in acquisition of resistance to chemotherapy. Cancer Cell 2002, 1, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Barbazuk, W.B.; Korf, I.; Kadavi, C.; Heyen, J.; Tate, S.; Wun, E.; Bedell, J.A.; McPherson, J.D.; Johnson, S.L. The syntenic relationship of the zebrafish and human genomes. Genome Res. 2000, 10, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- McAleer, M.F.; Davidson, C.; Davidson, W.R.; Yentzer, B.; Farber, S.A.; Rodeck, U.; Dicker, A.P. Novel use of zebrafish as a vertebrate model to screen radiation protectors and sensitizers. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Bladen, C.L.; Lam, W.K.; Dynan, W.S.; Kozlowski, D.J. DNA damage response and Ku80 function in the vertebrate embryo. Nucleic Acids Res. 2005, 33, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Daroczi, B.; Kari, G.; McAleer, M.F.; Wolf, J.C.; Rodeck, U.; Dicker, A.P. In vivo radioprotection by the fullerene nanoparticle DF-1 as assessed in a zebrafish model. Clin. Cancer Res. 2006, 12, 7086–7091. [Google Scholar] [CrossRef] [PubMed]
- Geiger, G.A.; Parker, S.E.; Beothy, A.P.; Tucker, J.A.; Mullins, M.C.; Kao, G.D. Zebrafish as a “Biosensor”? Effects of ionzing radiation and amifostine on embryonic viability and development. Cancer Res. 2006, 66, 8172–8181. [Google Scholar] [CrossRef] [PubMed]
- Yum, E.H.W.; Ng, C.K.M.; Lin, A.C.C.; Cheng, S.H.; Yu, K.N. Experimental setup for studying the effects of alpha particles on zebrafish embryos. Nucl. Instrum. Methods B 2007, 264, 171–176. [Google Scholar] [CrossRef]
- Yum, E.H.W.; Choi, V.W.Y.; Nikezic, D.; Li, V.W.T.; Cheng, S.H.; Yu, K.N. α-particle-induced bystander effects between zebrafish embryos in vivo. Radiat. Meas. 2009, 44, 1077–1080. [Google Scholar] [CrossRef]
- Yum, E.H.W.; Li, V.W.T.; Choi, V.W.Y.; Cheng, S.H.; Yu, K.N. Effects of alpha particles on zebrafish embryos. Appl. Radiat. Isot. 2010, 68, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Choi, V.W.Y.; Konishi, T.; Oikawa, M.; Iso, H.; Cheng, S.H.; Yu, K.N. Adaptive response in zebrafish embryos induced using microbeam protons as priming dose and X-ray photons as challenging dose. J. Radiat. Res. 2010, 51, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Choi, V.W.Y.; Lam, R.K.K.; Chong, E.Y.W.; Cheng, S.H.; Yu, K.N. Designing experimental setup and procedures for studying alpha-particle-induced adaptive response in zebrafish embryos in vivo. Nucl. Instrum. Methods B 2010, 268, 651–656. [Google Scholar] [CrossRef]
- Choi, V.W.Y.; Yu, K.N. Embryos of the zebrafish Danio rerio in studies of non-targeted effects of ionizing radiation. Cancer Lett. 2015, 356, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Choi, V.W.Y.; Wong, M.Y.P.; Cheng, S.H.; Yu, K.N. Effects of exogenous carbon monoxide on radiation-induced bystander effect in zebrafish embryos in vivo. Appl. Radiat. Isot. 2012, 70, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wu, L.; Chen, S.; Yu, K.N. Exogenous carbon monoxide protects the bystander Chinese hamster ovary cells in mixed co-culture system after alpha-particle irradiation. Carcinogenesis 2010, 31, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Magné, N.; Toillonb, R.-A.; Botteroc, V.; Didelotd, C.; van Houttea, P.; Gérarde, J.-P.; Peyronc, J.-F. NF-κB modulation and ionizing radiation: Mechanisms and future directions for cancer treatment. Cancer Lett. 2006, 231, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Volcic, M.; Karl, S.; Baumann, B.; Salles, D.; Daniel, P.; Fulda, S.; Wiesmüller, L. NF-κB regulates DNA double-strand break repair in conjunction with BRCA1-CtIP complexes. Nucleic Acids Res. 2012, 40, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, P.; Saintigny, Y.; Lopez, B.S. p53’s double life: Transactivation-independent repression of homologous recombination. Trends Genet. 2004, 20, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Voll, R.E.; Ghosh, S. Phosphorylation of NF-kB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1998, 1, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.A.; Perkins, N.D. Transcriptional cross talk between NF-κB and p53. Mol. Cell. Biol. 1999, 19, 3485–3495. [Google Scholar] [PubMed]
- Ahmed, K.M.; Li, J.J. NF-κB-mediated adaptive resistance to ionizing radiation. Free Radic. Biol. Med. 2008, 44, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Strair, R.K.; Gharibo, M.; Schaar, D.; Rubin, A.; Harrison, J.; Aisner, J.; Lin, H.-C.; Lin, Y.; Goodell, L.; Anand, M.; et al. Nuclear factor-κB modulation in patients undergoing induction chemotherapy for acute myelogenous leukemia. Clin. Cancer Res. 2008, 14, 7564–7568. [Google Scholar] [CrossRef] [PubMed]
- Courtois, G.; Smahi, A. NF-κB-related genetic diseases. Cell Death Differ. 2006, 13, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Gupta, S.; Kaur, S.; Ponnusamy, M.P.; Batra, S.K. Emerging trends for radioimmunotherapy in solid tumors. Cancer Biother. Radiopharm. 2013, 28, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Teulon, I.; Lozza, C.; Pèlegrin, A.; Vivès, E.; Pouget, J.P. General overview of radioimmunotherapy of solid tumors. Immunotherapy 2013, 5, 467–487. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Sgouros, G. Radioimmunotherapy of solid tumors: Searching for the right target. Curr. Drug Deliv. 2011, 8, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, V.E.; Waters, B.K.; Rossant, J. Interactions between diploid embryonal carcinoma cells and early embryonic cells. Cell Differ. 1984, 15, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Tzukerman, M.; Rosenberg, T.; Reiter, I.; Ben-Eliezer, S.; Denkberg, G.; Coleman, R.; Reiter, Y.; Skorecki, K. The influence of a human embryonic stem cell-derived microenvironment on targeting of human solid tumor xenografts. Cancer Res. 2006, 66, 3792–3801. [Google Scholar] [CrossRef] [PubMed]
- Durr, M.; Harder, F.; Merkel, A.; Bug, G.; Henschler, R.; Muller, A.M. Chimaerism and erythroid marker expression after microinjection of human acute myeloid leukaemia cells into murine blastocysts. Oncogene 2003, 22, 9185–9191. [Google Scholar] [CrossRef] [PubMed]
- Hochedlinger, K.; Blelloch, R.; Brennan, C.; Yamada, Y.; Kim, M.; Chin, L.; Jaenisch, R. Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev. 2004, 18, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.C.; Huang, C.C.; Chen, C.I.; Liu, C.H.; Hsieh, Y.S.; Huang, C.Y.; Lee, M.S.; Liu, J.Y. Leukemia inhibitory factor antisense oligonucleotide inhibits the development of murine embryos at preimplantation stages. Biol. Reprod. 2004, 70, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Yang, Z.; Qin, H. The relationship between early embryo development and tumourigenesis. J. Cell. Mol. Med. 2010, 14, 2697–2701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yeh, J.R.; Mara, A.; Ju, R.; Hines, J.F.; Cirone, P.; Griesbach, H.L.; Schneider, I.; Slusarski, D.C.; Holley, S.A.; et al. A chemical and genetic approach to the mode of action of fumagillin. Chem. Biol. 2006, 13, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Pugach, E.K.; Hettmer, S.; Storer, N.Y.; Liu, J.; DiBiase, A.; Zon, L.I.; Chen, E.Y.; Ignatius, M.S.; Poss, K.D.; et al. A novel chemical screening strategy in zebrafish identifies common pathways in embryogenesis and rhabdomyosarcoma development. Development 2013, 140, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, R.K.K.; Fung, Y.K.; Han, W.; Yu, K.N. Rescue Effects: Irradiated Cells Helped by Unirradiated Bystander Cells. Int. J. Mol. Sci. 2015, 16, 2591-2609. https://doi.org/10.3390/ijms16022591
Lam RKK, Fung YK, Han W, Yu KN. Rescue Effects: Irradiated Cells Helped by Unirradiated Bystander Cells. International Journal of Molecular Sciences. 2015; 16(2):2591-2609. https://doi.org/10.3390/ijms16022591
Chicago/Turabian StyleLam, R. K. K., Y. K. Fung, W. Han, and K. N. Yu. 2015. "Rescue Effects: Irradiated Cells Helped by Unirradiated Bystander Cells" International Journal of Molecular Sciences 16, no. 2: 2591-2609. https://doi.org/10.3390/ijms16022591
APA StyleLam, R. K. K., Fung, Y. K., Han, W., & Yu, K. N. (2015). Rescue Effects: Irradiated Cells Helped by Unirradiated Bystander Cells. International Journal of Molecular Sciences, 16(2), 2591-2609. https://doi.org/10.3390/ijms16022591





_Kwan_Ngok_Yu.png)