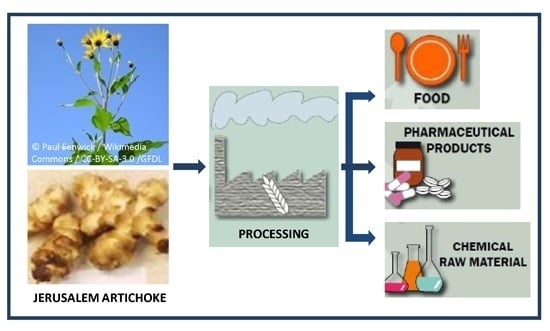
Economically Viable Components from Jerusalem Artichoke (Helianthus tuberosus L.) in a Biorefinery Concept
Abstract
:1. Introduction—Characteristics of the Jerusalem Artichoke for Potential Biorefinery or Multipurpose Use
2. Carbohydrates—Types, Content and Potential Uses
3. Proteins—Types, Content and Potential Uses
| Clone | First Harvest (9 September 2011) | Second Harvest (14 October 2011) | Third Harvest (7 December 2011) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | Tubers | Leaves | Tubers | Leaves | Tubers | |||||||||||||
| 1 | 18.6 | ± | 0.13 | 6.19 | ± | 0,09 | 16.6 | ± | 0.04 | 8.56 | 8.75 | 9.28 | ± | 1.90 | ||||
| 2 | 22.4 | ± | 0.04 | 6.75 | ± | 0.09 | 16.2 | ± | 1.77 | 6.75 | ± | 0.00 | 8.06 | ± | 2.12 | 8.00 | ± | 1.32 |
| 3 | 23.7 | ± | 0.27 | 8.31 | ± | 0.09 | 21.3 | ± | 0.40 | 5.91 | ± | 0.04 | 11.3 | ± | 5.70 | 6.47 | ± | 0.75 |
| 4 | 16.6 | ± | 0.09 | 8.50 | ± | 0.00 | 20.8 | ± | 0.84 | 8.69 | ± | 0.18 | 7.19 | ± | 2.65 | 7.19 | ± | 2.48 |
| 5 | 16.3 | ± | 0.13 | 8.44 | ± | 0.00 | 8.75 | ± | 0.18 | 6.75 | ± | 0.27 | n.d. | 6.69 | ± | 0.80 | ||
| 6 | 16.2 | ± | 0.18 | 7.88 | ± | 0.27 | 9.84 | ± | 0.57 | 5.25 | ± | 0.00 | 7.94 | ± | 2.83 | 7.12 | ± | 3.01 |
| 7 | 19.2 | ± | 0.31 | 9.38 | ± | 0.53 | 17.0 | ± | 0.35 | 5.91 | ± | 0.04 | 7.50 | ± | 2.03 | 5.34 | ± | 0.22 |
| 8 | 24.5 | ± | 0.40 | n.d. | 21.3 | ± | 1.15 | 7.03 | ± | 0.13 | 7.12 | ± | 2.21 | 6.78 | ± | 1.02 | ||
| 9 | 18.3 | ± | 0.04 | n.d. | 10.5 | ± | 0.22 | 6.62 | ± | 0.09 | 10.3 | ± | 4.42 | 7.18 | ± | 0.62 | ||
| 10 | 16.9 | ± | 0.09 | 7.44 | ± | 0.09 | 16.4 | ± | 0.44 | 6.94 | 9.25 | ± | 4.33 | 6.06 | ± | 0.09 | ||
| 11 | 18.3 | ± | 0.00 | 7.31 | ± | 0.00 | 16.6 | ± | 1.50 | 8.06 | ± | 0.09 | 7.94 | ± | 5.04 | 6.47 | ± | 0.84 |
4. Bioactive Compounds—Type, Content and Potential Uses
| Clone | First Harvest (9 September 2011) | Second Harvest (14 October 2011) | Third Harvest (7 December 2011) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | Tubers | Leaves | Tubers | Leaves | Tubers | |||||||||||||
| 1 | 44.0 | ± | 0.96 | 10.8 | ± | 0.65 | 28.3 | 5.30 | ± | 0.11 | 0.60 | ± | 0.03 | 1.63 | ± | 0.20 | ||
| 2 | 39.0 | ± | 0.99 | 8.16 | ± | 0.37 | 15.9 | ± | 1.38 | 8.14 | ± | 0.43 | 0.95 | ± | 0.05 | 3.57 | ± | 0.24 |
| 3 | 41.6 | ± | 2.12 | 11.8 | ± | 0.80 | n.d. | 5.85 | ± | 0.18 | 1.04 | ± | 0.03 | 1.25 | ± | 0.05 | ||
| 4 | 37.6 | ± | 4.27 | 11.6 | ± | 1.30 | 17.4 | ± | 0.12 | 8.94 | ± | 0.18 | 1.99 | ± | 0.06 | n.d. | ||
| 5 | 37.8 | ± | 2.61 | 7.79 | ± | 1.70 | 12.7 | ± | 0.96 | 6.14 | ± | 3.68 | 0.43 | ± | 0.05 | n.d. | ||
| 6 | 36.8 | ± | 4.79 | 11.5 | ± | 0.61 | 38.6 | ± | 0.98 | 10.0 | ± | 0.48 | 0.51 | ± | 0.16 | 3.34 | ||
| 7 | 42.9 | ± | 2.44 | 10.6 | ± | 1.20 | 37.5 | ± | 0.94 | 9.91 | ± | 0.60 | 1.13 | ± | 0.02 | 2.92 | ||
| 8 | 47.2 | ± | 1.36 | 10.8 | ± | 0.51 | 14.9 | ± | 0.91 | 9.12 | ± | 0.10 | 1.17 | ± | 0.04 | 2.91 | ||
| 9 | 43.4 | ± | 2.60 | 9.99 | ± | 0.70 | 22.5 | ± | 1.62 | 5.31 | ± | 0.11 | n.d. | 3.19 | ||||
| 10 | 39.2 | ± | 1.76 | 6.55 | ± | 0.24 | 19.3 | ± | 0.52 | 5.35 | ± | 0.31 | 0.58 | ± | 0.04 | 2.23 | ||
| 11 | 42.6 | ± | 3.06 | 11.9 | ± | 0.67 | 22.8 | ± | 0.69 | 9.51 | ± | 0.01 | 2.36 | ± | 0.04 | 2.02 | ||
5. Economic Aspects of Jerusalem Artichoke Cultivation as a Biorefinery Crop
6. Issues Related to the Multipurpose Use of Crops
7. Preliminary Economic Analyses of the Use of Jerusalem Artichoke as a Biorefinery Crop
| Parameter | Unit | Low | High | References |
|---|---|---|---|---|
| Protein extraction efficiency | [%] | 37 | 80 | [85,86] |
| Rubisco fraction of protein | [%] | 4 | 28 | [46] |
| Rubisco purification efficiency | [%] | 80 | 90 | own assumption |
| Sugar hydrolisation efficiency | [%] | 89 | 95 | [30,87] |
| Succinic acid yield | [%] | 67 | 74 | [30] |
| Parameter | Methane Potential |
|---|---|
| [Nm3/MgVS] | |
| Residual sugar in tubers a | 378 |
| Proteins [89] | 516 |
| Lipids [90] | 1026 |
| Hemicellulose [91] | 430 |
| Cellulose | 420 |
| Extractives | 400 |
| Uronic acid | 292 |
| Product | Unit | Processing Costs | Income | References | ||
|---|---|---|---|---|---|---|
| Low | High | Low | High | |||
| Methane a | [€/MWh] | 41 | 49 | 84 | 87 | [93,94] |
| Protein extraction | [€/Mg] | 200 | 200 | 5500 | 11,000 | Income data based on market price analyses |
| Rubisco extraction | [€/Mg] | 200 | 200 | 16,500 | 33,000 | Income data tripled from mixed protein extract |
| Succinic acid | [€/Mg] | 365 | 707 | 912 | 4561 | [95,96] |


8. Conclusions—Can Jerusalem Artichoke Be Seen as a Potential Biorefinery Crop?
Acknowledgments
Author Contributions
Definitions
Conflicts of Interest
References
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Cherubini, F.; Sergio, U. Crop residues as raw materials for biorefinery systems—A LCA case study. Appl. Energy 2010, 87, 47–57. [Google Scholar] [CrossRef]
- Kamm, B.; Kamm, M. Principles of biorefineries. Appl. Microbiol. Biotechnol. 2004, 64, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.; Elias, R.; Fish, S.; Fowler, P.; Gallagher, J. The biorefining opportunities in Wales: Understanding the scope for building a sustainable, biorenewable economy using plant biomass. Chem. Eng. Res. Des. 2009, 87, 1147–1161. [Google Scholar] [CrossRef]
- Srinivasan, S. The food v. fuel debate: A nuanced view of incentive structure. Renew. Energy 2009, 34, 950–954. [Google Scholar] [CrossRef]
- Rathmann, R.; Szklo, A.; Schaeffer, R. Land use competition for production of food and liquid biofuels: An analysis of the arguments in the current debate. Renew. Energy 2010, 35, 14–22. [Google Scholar] [CrossRef]
- Bergh, J.; Freeman, M.; Sigurdsson, B.; Kellomäki, S.; Laitinen, K.; Niinistö, S.; Peltola, H.; Linder, S. Modelling the short-term effects of climate change on the productivity of selected tree species in Nordic countries. For. Ecol. Manag. 2003, 183, 327–340. [Google Scholar] [CrossRef]
- Ruttanaprasert, R.; Baterng, P.; Jogloy, S.; Vorasoot, N.; Kesmala, T.; Kanwar, R.S.; Holbrook, C.C.; Patanothai, A. Genotypic variability for tuber yield, biomass, and drought tolerance in Jerusalem artichoke germplasm. Turk. J. Agric. For. 2014, 38, 570–580. [Google Scholar] [CrossRef]
- Yang, L.; He, Q.S.; Corscadden, K.; Udenigwe, C.C. The prospects of Jerusalem artichoke in functional food ingredients and bioenergy production. Biotechnol. Rep. 2015, 5, 77–88. [Google Scholar] [CrossRef]
- Ma, X.Y.; Zhang, L.H.; Shao, H.B.; Zhang, F.; Ni, F.T.; Brestic, M. Jerusalem artichoke (Helianthus tuberosus), a medicinal salt-resistant plant has high adaptability and multiple-use values. J. Med. Plants Res. 2011, 5, 1272–1279. [Google Scholar]
- Slimestad, R.; Seljaasen, R.; Meijer, K.; Skar, S.L. Norwegian-grown Jerusalem artichoke (Helianthus tuberosus L.): Morphology and content of sugars and fructo-oligosaccharides in stems and tubers. J. Sci. Food Agric. 2010, 90, 956–964. [Google Scholar] [PubMed]
- Gunnarsson, I.B.; Svensson, S.-E.; Johansson, E.; Karakashev, D.; Angelidaki, I. Potential of Jerusalem artichoke (Helianthus tuberosus L.) as a biorefinery crop. Ind. Crops Prod. 2014, 56, 231–240. [Google Scholar] [CrossRef]
- Godin, B.; Lamaudière, S.; Agneessens, R.; Schmit, T.; Goffart, J.-P.; Stilmant, D.; Gerin, P.A.; Delcarte, J. Chemical characteristics and biofuel potential of several vegetal biomasses grown under a wide range of environmental conditions. Ind. Crops Prod. 2013, 48, 1–12. [Google Scholar] [CrossRef]
- Liu, X.-X.; Han, L.-P.; Steinberger, Y.; Xie, G.-H. Genetic variation and yield performance of Jerusalem artichoke germplasm collected in China. Agric. Sci. China 2011, 10, 668–678. [Google Scholar] [CrossRef]
- Cereal Yield (kg per hectare). Available online: http://data.worldbank.org/indicator/AG.YLD.CREL.KG (assessed on 19 March 2015).
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Bowles, D. Plant production systems for bioactive small molecules. Curr. Opin. Biotechnol. 2012, 23, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kays, S.J.; Nottingham, S.F. Classification, identification and distribution. In Biology and Chemistry of Jerusalem Artichoke; CRC Press: Boca Raton, FL, USA, 2007; pp. 29–34. [Google Scholar]
- Kays, S.J.; Nottingham, S.F. Pollinators, pests and diseases. In Biology and Chemistry of Jerusalem Artichoke; CRC Press: Boca Raton, FL, USA, 2007; pp. 365–382. [Google Scholar]
- Kays, S.J.; Nottingham, S.F. Developmental biology, resource allocation, and yield. In Biology and Chemistry of Jerusalem Artichoke; CRC Press: Boca Raton, FL, USA, 2007; pp. 269–364. [Google Scholar]
- Kosaric, N.; Cosentino, G.P.; Wieczorek, A.; Duvnjak, Z. The Jerusalem artichoke as an agricultural crop. Biomass 1984, 5, 1–36. [Google Scholar] [CrossRef]
- Barclay, T.; Ginic-Markovic, M. Inulin: A versatile polysaccharide with multiple pharmaceutical and food chemical uses. J. Excip. Food Chem. 2010, 1, 27–50. [Google Scholar]
- Kaur, N.; Gupta, A.K. Applications of inulin and loigofructose in health and nutrition. J. Biosci. 2002, 27, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Flamm, G.; Glinsmann, W.; Kritchevsky, D.; Prosky, L.; Roberfroid, M. Inulin and oligofructose as dietary fiber: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2001, 41, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Baldini, M.; Danusco, F.; Turi, M.; Vannozzi, G.P. Evaluation of new clones of Jerusalem artichoke (Helianthus tuberosus L.) for inulin and sugar yield from stalks and tubers. Ind. Crops Prod. 2004, 19, 25–40. [Google Scholar] [CrossRef]
- Franck, A. Technological functionality of inulin and oligofructose. Br. J. Nutr. 2002, 87, S287–S291. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Jain, H.; Mann, P.; Gupta, A.K.; Singh, R. A comparison of properties of invertases and inulinase from chicory. Plant Physiol. Biochem. 1992, 30, 445–450. [Google Scholar]
- Collins, M.; McCoy, J.E. Chicory productivity, forage quality, and response to nitrogen fertilization. Agron. J. 1997, 89, 232–238. [Google Scholar] [CrossRef]
- Szambelan, K.; Nowak, J.; Czarnecki, Z. Use of Zymonas mobilis and Saccharomyces cerevisiae mixed with Kluyveromyces fragilis for improved ethanol production from Jerusalem artichoke tubers. Biotechnol. Lett. 2004, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, I.B.; Karakashev, D.; Angelidaki, I. Succinic acid production by fermentation of Jerusalem artichoke tuber hydrolysate with Actinobacillus succinogenes 130Z. Ind. Crops Prod. 2014, 62, 125–129. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Wang, Y.; Du, Y.; Qin, S. Biorefinery products from the inulin-containing crop Jerusalem artichoke. Biotechnol. Lett. 2013, 35, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Godin, B.; Lamaudière, S.; Agneessens, R.; Schmit, T.; Goffart, J.-P.; Stilmant, D.; Gerin, P.A.; Delcarte, J. Chemical characteristics and biofuels potentials of various plant biomasses: Influence of the harvesting date. J. Sci. Food Agric. 2013, 13, 3216–3224. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.L.; Kim, C.H. Ethanol production using whole plant biomass of Jerusalem artichoke by Kluyveromyces marxianus CBS1555. Appl. Biochem. Biotechnol. 2013, 169, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P.K.; Bajpai, P. Cultivation and utilization of Jerusalem artichoke for ethanol, single cell protein, and high-fructose syrup production. Enzym. Microb. Technol. 1991, 13, 359–362. [Google Scholar] [CrossRef]
- Newson, W.R.; Kuktaite, R.; Hedenqvist, M.S.; Gällstedt, M.; Johansson, E. Effect of additives on the tensile performance and protein solubility of industrial oilseed residual based plastics. J. Agric. Food Chem. 2014, 62, 6707–6715. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, E.; Gebusia, A.; Florkiewicz, A.; Mickowska, B. The content of protein and of amino acids in Jerusalem artichoke tubers (Helianthus tuberosus L.) of red variety Rote zonenkugel. Acta Sci. Pol. Technol. Aliment. 2011, 10, 433–441. [Google Scholar] [PubMed]
- Blomfeldt, T.O.J.; Olsson, R.T.; Menon, M.; Plackett, D.; Johansson, E.; Hedenqvist, M. Novel foams based on freeze-dried renewable vital wheat gluten. Macromol. Mater. Eng. 2010, 295, 796–801. [Google Scholar] [CrossRef]
- Kuktaite, R.; Plivelic, T.S.; Cerenius, Y.; Hedenqvist, M.S.; Gällstedt, M.; Marttila, S.; Ignell, R.; Popineau, Y.; Tranquet, O.; Shewry, P.R.; et al. Structure and morphology of wheat gluten films: From polymeric protein aggregates towards superstructure arrangements. Biomacromolecules 2011, 12, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Malik, A.H.; Hussain, A.; Rasheed, F.; Newson, W.R.; Plivelic, T.; Hedenqvist, M.S.; Gällstedt, M.; Kuktaite, R. Wheat gluten polymer structures: The impact of genotype, environment and processing on their functionality in various applications. Cereal Chem. 2013, 90, 367–376. [Google Scholar] [CrossRef]
- Rasheed, F.; Newson, R.W.; Plivelic, T.S.; Kuktaite, R.; Hedenqvist, M.S.; Gällstedt, M.; Johansson, E. Structural architecture and solubility of native and modified gliadin and glutenin proteins: Non-crystalline molecular and atomic organizations. RSC Adv. 2014, 4, 2051–2060. [Google Scholar] [CrossRef]
- Johansson, E.; Kuktaite, R.; Andersson, A.; Prieto-Linde, M.L. Protein polymer built-up during wheat development: Influences of temperature and nitrogen timing. J. Sci. Food Agric. 2005, 85, 473–479. [Google Scholar] [CrossRef]
- Newson, W.R.; Rasheed, F.; Kuktaite, R.; Hedenqvist, M.S.; Gällstedt, M.; Plivelic, T.S.; Johansson, E. Commercial potato protein concentrate as a novel source for thermoformed bio-based plastic films with unusual polymerization and tensile properties. RSC Adv. 2015, 5, 32217–32226. [Google Scholar] [CrossRef]
- Scott, E.; Peter, F.; Sanders, J. Biomass in the manufacture of industrial products—The use of proteins and amino acids. Appl. Microbiol. Biotechnol. 2007, 75, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shen, S. Effective protein extraction protocol for proteomics studies of Jerusalem artichoke leaves. J. Sep. Sci. 2013, 36, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, M.-D.; Shen, S.-H. Comparison of protein extraction methods suitable for proteomics analysis in seedling roots of Jerusalem artichoke under salt (NaCl) stress. Afr. J. Biotechnol. 2011, 10, 7650–7657. [Google Scholar]
- Raven, J.A. Rubisco: Still the most abundant protein of Earth? New Phytol. 2013, 198, 1–3. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.; Nieuwland, M. TNO Report V9436 Literature study on the properties of Rubisco. Available online: http://www.dutchbiorefinerycluster.nl/download/298/documenten/Literature_report_rubisco.pdf (accessed on 16 January 2014).
- Pouvreau, L.; Smit, B.; van de Velde, F. Securing food proteins: From by-products to functional ingredient. In Gums and Stabilisers for the Food Industry 17. The Changing Face of Food Manufacture: The Role of Hydrocolloids; Williams, P.A., Phillips, G.O., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2014; pp. 46–51. [Google Scholar]
- Van de Velde, F.; Alting, A.C.; Pouvreau, L. Process for Isolating a Dechlorophyllized Rubisco Preparation from a Plant Material. Patent number: WO2011078671, 30 June 2011. [Google Scholar]
- Lamsal, B.P.; Koegel, R.G.; Gunasekaran, S. Some physicochemical and functional properties of alfalfa soluble leaf proteins. LWT Food Sci. Technol. 2007, 40, 1520–1526. [Google Scholar] [CrossRef]
- Cho, S.-W.; Gällstedt, M.; Johansson, E.; Hedenqvist, M.S. Injection-molded nanocomposites and materials based on wheat gluten. Int. J. Biol. Macromol. 2011, 48, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Türe, H.; Gällstedt, M.; Kuktaite, R.; Johansson, E.; Hedenqvist, M.S. Protein network structure and properties of wheat gluten extrudates using a novel solvent-free approach with urea as a comined denaturant and plasticizer. Soft Matter 2011, 7, 9416–9423. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Q.; Bjork, L.; Trajkovski, V.; Uggla, M. Evaluation of antioxidant activities of rosehip ethanol extracts in different test systems. J. Sci. Food Agric. 2000, 80, 2021–2027. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Saura-Calixto, F. Literature data may underestimate the actual antioxidant capacity of cereals. J. Agric. Food Chem. 2005, 53, 5036–5040. [Google Scholar] [CrossRef] [PubMed]
- Widén, C.; Ekholm, A.; Piwowar-Zail, D.; Rumpunen, K. Antioxidant activity of polyphenol rich fruits on human erythrocytes. Acta Hort. 2012, 926, 669–674. [Google Scholar]
- Johansson, E.; Hussain, A.; Kuktaite, R.; Andersson, S.C.; Olsson, M.E. Contribution of organically grown crops to human health. Int. J. Environ. Res. Public Health 2014, 11, 3870–3893. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.C.; Rumpunen, K.; Johansson, E.; Olsson, M.E. Carotenoid content and composition in rose hips (Rosa spp.) during ripening, determination of suitable maturity marker and implications for health promoting food products. Food Chem. 2011, 128, 689–696. [Google Scholar] [CrossRef]
- Andersson, S.C.; Olsson, M.E.; Gustavsson, K.-E.; Johansson, E.; Rumpunen, K. Tocopherols in rose hips (Rosa spp.) during ripening. J. Sci. Food Agric. 2012, 92, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- James, A.D. Handbook of energy crops. Available online: http://www.hort.purdue.edu/newcrop/duke_energy/Helianthus_tuberosus.html (accessed on 6 March 2015).
- Yuan, X.; Gao, M.; Xiao, H.; Tan, C.; Du, Y. Free radical scavenging activities and bioactive substances of Jerusalem artichoke (Helianthus tuberosus L.) leaves. Food Chem. 2012, 133, 10–14. [Google Scholar] [CrossRef]
- Cabello-Hurtado, F.; Durst, F.; Jorrin, J.V.; Werck-Reichhart, D. Coumarins in Helianthus tuberosus: Characterization, induced accumulation and biosynthesis. Phytochemistry 1998, 49, 1029–1036. [Google Scholar] [CrossRef]
- Yoshihara, T.; Matsuura, H.; Ichihara, A.; Kikuta, Y.; Koda, Y. Tuber forming substances of Jerusalem artichoke (Helianthus tuberosus L.). Curr. Plant Sci. Biotechnol. Agric. 1992, 13, 286–290. [Google Scholar]
- Matsuura, H.; Yoshihara, T.; Ichihara, A. Four new polyacetylenic glucosides, methyl beta-d-glucopyranosyl helianthenate C-F, from Jerusalem artichoke (Helianthus tuberosus L.). Biosci. Biotechnol. Biochem. 1993, 57, 1492–1498. [Google Scholar] [CrossRef]
- Pan, L.; Sinden, M.R.; Kennedy, A.H.; Chai, H.; Watson, L.E.; Graham, T.L.; Kinghorn, A.D. Bioactive constituents of Helianthus tuberosus (Jerusalem artichoke). Phytochem. Lett. 2009, 2, 15–18. [Google Scholar] [CrossRef]
- Ahmed, M.S.; El-Sakhawy, F.S.; Soliman, S.N.; Abou-Hussein, D.M.R. Phytochemical and biological study of Helianthus tuberosus L. Egypt J. Biomed. Sci. 2005, 18, 134–147. [Google Scholar]
- Chen, F.; Long, X.; Yu, M.; Liu, Z.; Liu, L.; Hongbo, S. Phenolics and antifungal activities analysis in industrial crop Jerusalem artichoke (Helianthus tuberosus L.) leaves. Ind. Crops Prod. 2013, 47, 339–345. [Google Scholar] [CrossRef]
- Yuan, X.; Cheng, M.; Gao, M.; Zhuo, R.; Zhang, L.; Xiao, H. Cytotoxic constituents from the leaves of Jerusalem artichoke (Helianthus tuberosus L.) and their structure-activity relationships. Phytochem. Lett. 2013, 6, 21–25. [Google Scholar] [CrossRef]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Mielmann, A. The utilization of Lucerne (Medicago sativa): A review. Br. Food J. 2013, 115, 590–600. [Google Scholar] [CrossRef]
- Innovation in Europe: Research and Results. Available online: http://ec.europa.eu/research/success/en/agr/0331e.html (assessed on 7 March 2015).
- Ethanol 2005–2015. Available online: http://www.tradingeconomics.com/commodity/ethanol (assessed on 7 March 2015).
- NNFCC Platform Chemicals. Renewable Chemicals Factsheet. Available online: http://www.nnfcc.co.uk (accessed on 7 March 2015).
- Trading Economics. Available online: http://www.tradingeconomics.com/commodity/naturalgas (assessed on 7 March 2015).
- The CNG-CBG White Paper. Compressed Natural Gas and Compressed Biogas. An Overview of the Technology, Economics and Project Development Process. Available online: http://www.americanbiogascouncil.org (assessed on 7 March 2015).
- Inulin, Inulin Suppliers and manufacturers at Alibaba.com. Available online: http://www.alibaba.com/showroom/inulin.html (assessed on 7 March 2015).
- Hermann, B.G.; Blok, K.; Patel, M.K. Producing bio-based bulk chemicals using industrial biotechnology saves energy and combats climate change. Environ. Sci. Technol. 2007, 41, 7915–7921. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-L.; Shao, R.; Dong, R.; Yun, Z. An integrated approach for obtaining biodiesel, sterols, gossypol, and raffinose from cottonseed on a biorefinery concept. Energy 2014, 70, 149–158. [Google Scholar] [CrossRef]
- Lapkin, A.A.; Plucinski, P.K.; Cutler, M. Comparative assessment of technologies for extraction of Artemisinin. J. Nat. Prod. 2006, 69, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Osborne, T.B. The proteins of the wheat kernel. Carnegie Int. Wash. Publ. 1907, 84, 1–119. [Google Scholar]
- Newson, W.; Johansson, E.; et al. Influences of protein extraction on film properties of Crambe abyssinica. (Manuscript in preparation).
- Gunnarsson, I.B.; Alvarado-Morales, M.; Angelidaki, I. Utilization of CO2 fixating bacterium Actinobacillus succinogenes 130Z for simultaneous biogas upgrading and biosuccinic acid production. Environ. Sci. Technol. 2014, 48, 12464–12468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojilla-Evangelista, M.P. Sequential Extraction Processing: Alternate Technology for Corn Wet Milling. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1990. Available online: http://www.orbichem.com/Chem-Netfacts.aspx?P_ID=208 (accessed on 21 April 2015). [Google Scholar]
- Ratanapariyanuch, K.; Tyler, R.T.; Shim, Y.Y.; Reaney, M.J.T. Biorefinery process for protein extraction from oriental mustard (Brassica juncea (L.) Czern.) using ethanol stillage. AMB Express 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Pekic, B.; Slavica, B.; Lepojevic, Z.; Petrovic, S.M. Effect of pH on the acid hydrolysis of Jerusalem artichoke inulin. Food Chem. 1985, 17, 169–173. [Google Scholar] [CrossRef]
- Symons, G.E.; Buswell, A.M. The methane fermentation of carbohydrates. J. Am. Chem. Soc. 1933, 55, 2028–2036. [Google Scholar] [CrossRef]
- Torabizadeh, H. All proteins have a basic molecular formula. Int. Sch. Sci. Res. Innov. 2011, 5, 777–781. [Google Scholar]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Preece, I.A. Studies on hemicelluloses: The hemicelluloses of maize cobs. Biochem. J. 1930, 24, 59–66. [Google Scholar] [PubMed]
- Hansson, P.; Saltzmann, I.-L.; Bååth Jacobsson, S.; Petersson, P. Produktionskalkyler för växtodling—Efterkalkyler för år 2013—Södra Sverige; Hushållningssällskapen Kalmar-Kronoberg-Bleking: Kristianstad, Sweden, 2013. [Google Scholar]
- Dahlgren, S. Realiserbar biogaspotential I Sverige år 2030 genom rötning och förgasning; WSP: Stockholm, Sweden, 2013; Available online: http://www.energigas.se/ (assessed on 21 April 2015).
- Lantz, M.; Björnsson, L. Styrmedel för en ökad produktion av gödselbaserad biogas. En fallstudie för Skåne och Västra Götalands län; Lund University: Lund, Sweden, 2014; Available online: http://www.biogassyd.se/download/18.38228ad4143b351109719d5d/1395838154412/Styrmedel+f%C3%B6r+%C3%B6kad+prod+av+g%C3%B6dselbaserad+biogas+Lantz_2014_Rapport+90.pdf (assessed on 21 April 2015).
- Hermann, B.G.; Patel, M. Today’s and tomorrow’s bio-based bulk chemicals from white biotechnology. Appl. Biochem. Biotechnol. 2007, 136, 361–388. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lee, S.Y. Production of succinic acid by bacterial fermentation. Enzym. Microb. Technol. 2006, 39, 352–361. [Google Scholar] [CrossRef]
- Cukalovic, A.; Stevens, C.V. Feasibility of production methods for succinic acid derivatives: A marriage of renewable resources and chemical technology. Biofuels Bioprod. Biorefin. 2008, 2, 505–529. [Google Scholar] [CrossRef]
- McKinlay, J.B.; Laivenieks, M.; Schindler, B.D.; McKinlay, A.A.; Siddaramappa, S.; Challacombe, J.F.; Lowry, S.R.; Clum, A.; Lapidus, A.L.; Burkhart, K.B.; et al. A genomic perspective on the potential of Actinobacillus succinogenes for industrial succinate production. BMC Genomics 2010, 11. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, J.; Vieille, C.; Zeikus, J.G. Prospects for a bio-based succinate industry. Appl. Microbiol. Biotechnol. 2007, 76, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Orbichem. CHEM-NET facts-Maleic anhydride; Tecnon Orbichem: Croydon, UK, 2013; Available online: http://www.orbichem.com/Chem-Netfacts.aspx?P_ID=208 (assessed on 21 April 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johansson, E.; Prade, T.; Angelidaki, I.; Svensson, S.-E.; Newson, W.R.; Gunnarsson, I.B.; Hovmalm, H.P. Economically Viable Components from Jerusalem Artichoke (Helianthus tuberosus L.) in a Biorefinery Concept. Int. J. Mol. Sci. 2015, 16, 8997-9016. https://doi.org/10.3390/ijms16048997
Johansson E, Prade T, Angelidaki I, Svensson S-E, Newson WR, Gunnarsson IB, Hovmalm HP. Economically Viable Components from Jerusalem Artichoke (Helianthus tuberosus L.) in a Biorefinery Concept. International Journal of Molecular Sciences. 2015; 16(4):8997-9016. https://doi.org/10.3390/ijms16048997
Chicago/Turabian StyleJohansson, Eva, Thomas Prade, Irini Angelidaki, Sven-Erik Svensson, William R. Newson, Ingólfur Bragi Gunnarsson, and Helena Persson Hovmalm. 2015. "Economically Viable Components from Jerusalem Artichoke (Helianthus tuberosus L.) in a Biorefinery Concept" International Journal of Molecular Sciences 16, no. 4: 8997-9016. https://doi.org/10.3390/ijms16048997
APA StyleJohansson, E., Prade, T., Angelidaki, I., Svensson, S. -E., Newson, W. R., Gunnarsson, I. B., & Hovmalm, H. P. (2015). Economically Viable Components from Jerusalem Artichoke (Helianthus tuberosus L.) in a Biorefinery Concept. International Journal of Molecular Sciences, 16(4), 8997-9016. https://doi.org/10.3390/ijms16048997