2.1. Formulation Design and Characterization of HCT Hydrogel
In this study, we used HPMC as a gelling agent to develop chitosan hydrogels (named as HCT hydrogel) for PDI.
Table 1 shows the composition of HCT hydrogel containing chitosan, TBO, and different concentrations of HPMC. For comparison, TBO with chitosan and TBO alone were used as controls to evaluate the PDI efficacy of HCT hydrogel. These formulations were prepared with an intention to optimize the content of HPMC in HCT hydrogel and understand its influence on the mechanical properties and the antimicrobial effect.
Table 2 summarized the physical properties and texture parameters including hardness, adhesion, and compressibility of HTC hydrogel and a marketed topical antibiotic gel (ELYZOL
®). The viscosity of HCT hydrogel was increased when increasing the content of HPMC (from 0.25% to 3%,
w/
w) in HTC hydrogel. The presence of HPMC caused the viscosity significantly increased from 36.56 to 629.8 cps when comparing with those of the controls (4.95 and 4.59 cps). Among the formulations tested, HCT hydrogel (F-3) containing 1% (
w/
w) HPMC appeared to be the most comparable formulation to the marketed product (ELYZOL
®) regarding the viscosity.
Texture analysis provides deeper insight on the hydrogel properties and enables correlation to applicability of the formulation. The amount of gelling agent in a formulation is of great importance with respect to its textural properties. It is expected that hydrogel adhesiveness would be correlated to hydrogel bio-adhesiveness. The results obtained by texture analysis showed the same trend as those found in viscosity test. When we increased HPMC concentration to HCT hydrogel, the hardness, adhesiveness, as well as compressibility for hydrogels were increased. Minimum and maximum hardness, compressibility, and adhesiveness values were exhibited by formulations containing 0.25% and 3% (
w/
w) of HPMC, respectively. Similarly, Ferrari
et al. [
11] reported that the strength of HPMC gels increased as the polymer concentration was increased. These results demonstrate that the incorporation of HPMC could enforce the structure of HCT hydrogel. In addition, the F-1 HCT hydrogel containing 0.25% (
w/
w) HPMC exhibited relatively similar textural properties as compared to ELYZOL
®.
Table 1.
Formulation composition of HCT hydrogel.
Table 1.
Formulation composition of HCT hydrogel.
| Formulation | HPMC 1 (%, w/w) | Chitosan 2 (%, w/w) | TBO (μM) |
|---|
| F-1 | 0.25 | 0.25 | 20 |
| F-2 | 0.5 | 0.25 | 20 |
| F-3 | 1 | 0.25 | 20 |
| F-4 | 2 | 0.25 | 20 |
| F-5 | 3 | 0.25 | 20 |
Table 2.
The viscosity and texture parameters of HCT hydrogel containing various concentrations of HPMC in comparison with a marketed product (ELYZOL®).
Table 2.
The viscosity and texture parameters of HCT hydrogel containing various concentrations of HPMC in comparison with a marketed product (ELYZOL®).
| Formulation | Viscosity (cps) | Hardness (N) | Adhesiveness (N mm) | Compressibility (N mm) |
|---|
| TBO only a | 4.95 ± 0.37 | 0.13 ± 0.05 | 0.23 ± 0.03 | 0.56 ± 0.05 |
| Mixture of TBO & chitosan b | 4.59 ± 0.28 | 0.14± 0.02 | 0.25 ± 0.03 | 0.78 ± 0.03 |
| F-1 | 36.56 ± 1.50 | 0.18 ± 0.03 | 0.88 ± 0.03 | 1.46 ± 0.18 |
| F-2 | 46.64 ± 2.13 | 0.22 ± 0.02 | 1.89 ± 0.07 | 2.67 ± 0.59 |
| F-3 | 294.8 ± 20.6 | 0.56 ± 0.03 | 3.88 ± 0.12 | 5.33 ± 0.24 |
| F-4 | 495.4 ± 58.9 | 0.93 ± 0.06 | 6.07 ± 0.21 | 8.16 ± 0.49 |
| F-5 | 629.8 ± 113.6 | 1.67 ± 0.04 | 14.10 ± 0.33 | 13.89 ± 0.90 |
| ELYZOL® c | 187.4 ± 18.3 | 0.18 ± 0.02 | 0.95 ± 0.08 | 2.33 ± 0.49 |
Table 3.
Injectability of HCT hydrogel in various concentrations of HPMC in comparison with a marketed product (ELYZOL®).
Table 3.
Injectability of HCT hydrogel in various concentrations of HPMC in comparison with a marketed product (ELYZOL®).
| Formulation | Injectability (gw·min−1) |
|---|
| 200 (gw) | 500 (gw) |
|---|
| distilled water | 8.983 ± 0.682 | 15.909 ± 1.351 |
| TBO only | 8.053 ± 0.432 | 15.861 ± 1.642 |
| Mixture of TBO & chitosan | 7.241 ± 0.334 | 10.340 ± 1.031 |
| F-1 | 2.808 ± 0.132 | 8.849 ± 0.872 |
| F-2 | 0.151 ± 0.062 | 7.251 ± 0.703 |
| F-3 | 0.008 ± 0.001 | 0.012 ± 0.004 |
| F-4 | 0.0021 ± 0.002 | 0.0115 ± 0.003 |
| F-5 | N/A | 0.0013 ± 0.001 |
| ELYZOL® | 0.324 ± 0.160 | 10.124 ± 1.095 |
To evaluate the practical use in clinical application, we further quantify the injectability of this HCT hydrogel. A quantitative method for measuring the ease of injectability was performed by placing a fixed loading weight on the top of the plunger and the amount of hydrogel expelled from the syringe was then measured under one minute. As shown in
Table 3, the decreased injectability was associated with the increase in HPMC concentration regardless the loading force. The F-5 HCT hydrogel containing 3% (
w/
w) HPMC was the most difficult one to be expelled from the syringe. On a comparative basis, the injectibiltiy of F-1 and F-2 were acceptable when compared to ELYZOL
® under 200 and 500 g loading mass, respectively.
The effect of increasing HPMC concentration in the formulation on the hardness, compressibility, and injectability of the gel also related to product viscoelasticity. Increased product viscoelasticity, particularly elasticity, offered an increased resistance to product deformation in texture and injectability, resulting in an increase in product hardness and work required for product compression/expulsion.
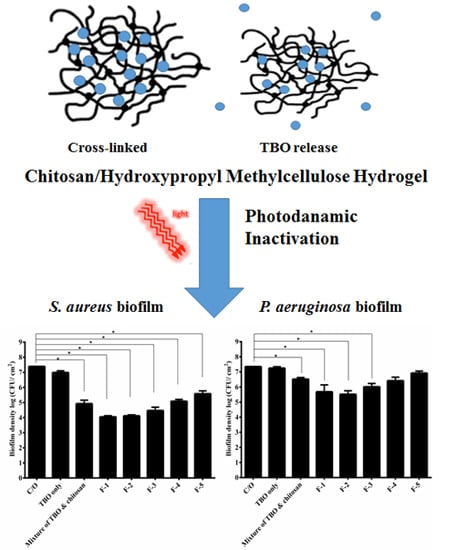
2.2. Assessment of PDI Efficacy of HTC Hydrogels against S. aureus and P. aeruginosa Biofilms
PDI efficacy of these HCT hydrogels was investigated against the biofilm cells of
S.
aureus and
P.
aeruginosa. As shown in
Figure 1A, approximately 1-log
10 reduction in viable counts was found in
S.
aureus biofilm cells incubated with TBO, while the mixture of TBO and chitosan showed a pronounced antimicrobial effect (~3.5-log reduction) under a light dose of 20 J·cm
−2 (
Figure 1A). The F-1 and F-2 HCT hydrogel also caused similar PDI efficacy as found in the mixture of TBO and chitosan. However, a significant decrease (
p < 0.05) in the PDI efficacy was observed when the HPMC concentration increased to 1% (
w/
w) or above. The survival fraction approximately reduced from 3- to 1-log when treated with F-1 to F-5, respectively. These results indicated that higher concentration of HPMC could increase the viscosity of hydrogel, but might constrain TBO release and decrease the PDI efficacy of HTC hydrogel. Similar results were also found in
P. aeruginosa biofilm treated with HCT hydrogel (
Figure 1B). HCT hydrogels (F-1, F-2 and F-3) mediated PDI resulted in a 1- to 2-log bacterial killing in the biofilm cells of
P. aeruginosa compared to 3-log killing treated with mixture of TBO and chitosan (
Figure 1B). The lower PDI efficacy in
P. aeruginosa is expected. Lipopolysaccharides are a major constituent of the
P.
aeruginosa membrane, and changes observed in membrane structure may result in changes to the drug permeability barrier [
12].
Figure 1.
Cell survival fraction of S. aureus (A) and P. aeruginosa (B) biofilm treated with HCT hydrogels-mediated PDI. Biofilm cells were incubated with 20 μM TBO or HCT hydrogels containing 20 μM TBO for 1 h, followed by light exposure at 20 J·cm−2. Each value is the mean from three independent experiments ± standard deviation. * p < 0.05.
Figure 1.
Cell survival fraction of S. aureus (A) and P. aeruginosa (B) biofilm treated with HCT hydrogels-mediated PDI. Biofilm cells were incubated with 20 μM TBO or HCT hydrogels containing 20 μM TBO for 1 h, followed by light exposure at 20 J·cm−2. Each value is the mean from three independent experiments ± standard deviation. * p < 0.05.
2.3. Penetration of TBO into Biofilms
It has been shown that the drug release is diffusion-controlled and depends mostly on the viscosity of the hydrogel formed [
13]. In addition, Ford
et al. have reported that the drug release was slowly in high viscosity HPMC gelling agents [
14,
15]. To verify whether higher HPMC concentration constrains TBO release, confocal microscopy analysis was performed to characterize the penetration of TBO into viable
S.
aureus biofilms.
Figure 2 shows the X–Z confocal images obtained from biofilms treated with TBO only, mixture of TBO and chitosan and HCT hydrogels containing different concentrations of HPMC, respectively. As expected, the overall fluorescence intensity and the penetration depth obtained from biofilm treated with TBO or the mixture of TBO and chitosan was significantly stronger than those of treated with HCT hydrogels.
Figure 2.
Confocal fluorescence imaging (X–Z) of S. aureus biofilms treated with HCT hydrogel for 1 h. The fluorescent signal from the TBO only biofilm was of higher intensity and extended deeper than 115 μm.
Figure 2.
Confocal fluorescence imaging (X–Z) of S. aureus biofilms treated with HCT hydrogel for 1 h. The fluorescent signal from the TBO only biofilm was of higher intensity and extended deeper than 115 μm.
The quantitative analysis as shown in
Table 4 demonstrated a significant decrease of TBO in deeper layers of the biofilm treated with HTC hydrogels. In the group treated with TBO solution, the fluorescent signal extended to a depth of ~110 μm, whereas the signals only extended to a depth in a range of 10 to 83 µm in the specimen treated with HCT hydrogels. These results indicate that the increase in the HPMC concentration of HTC hydrogel indeed constrains TBO release into the deeper layer of
S. aureus biofilm, which further explain the reduced PDI susceptibility of biofilms treated with hydrogel containing higher concentration of HPMC.
For clinical practice, PDI is more-easily carried out for localized infection. In this regards, a delivery system with good adhesion property is required to apply photosensitizer onto the infected tissues. Longer retention in the absorption site allows the photosensitizer to have a chance to bind and penetrate the biofilm. In this study, incorporation of HPMC offered a better adhesion property for this topical dosage form. However, optimizing HPMC concentration is necessary to obtain the optimized texture properties and PDI efficacy.
Table 4.
The average distribution depth of TBO into the biofilm. Values are mean ± standard deviation.
Table 4.
The average distribution depth of TBO into the biofilm. Values are mean ± standard deviation.
| Formulation | Average Depth (μm) |
|---|
| TBO only | >115 ± 15 |
| Mixture of TBO & chitosan | 110 ± 13 |
| F-1 | 83 ± 9 |
| F-2 | 52 ± 6 |
| F-3 | 35 ± 4 |
| F-4 | 18 ± 2 |
| F-5 | 10 ± 2 |
2.4. Influence of Tilt Degree on PDI
Based on the texture analysis, we have shown that high viscosity of dosage forms might provide a better adhesion on the treatment areas and prolong the drug contact (
Table 2). To mimic the clinical use, we tilted the plates containing biofilms and HCT hydrogel to 90 degrees for the following incubation, and then examined the PDI efficacy.
Figure 3 shows the survival rate of
S.
aureus and
P.
aeruginosa biofilms with tilt. Interestingly, the results were quite different from those shown in
Figure 1. Under tilt, there was no significant reduction in the survival rate of biofilm treated with the TBO. Treated with the mixture of TBO and chitosan approximately 2.5- and 0.7-log reductions were found against
S.
aureus and
P.
aeruginosa biofilm, respectively. Furthermore, F-1 and F-2 showed a significant better antimicrobial effect than the control against
S.
aureus and
P.
aeruginosa biofilm. Both approximately 3.5- and 2-log reductions in viable cell count were found against
S.
aureus and
P.
aeruginosa biofilm, respectively. The higher adhesive character contributed by increasing the incorporation of HPMC seems to provide a better contact with the biofilm and leading to a superior antimicrobial effect. However, the PDI efficacy decreased when the concentration of HPMC increased to 1% (
w/
w) or higher.
Figure 3.
Cell survival fraction S. aureus (A) and P. aeruginosa (B) biofilm incubated with various formulas of HCT hydrogel for 1 h under 90-degree tilt, and then subjected to 20 J·cm−2 of light illumination. Each value is the mean from three independent experiments ± standard deviation. * p < 0.05.
Figure 3.
Cell survival fraction S. aureus (A) and P. aeruginosa (B) biofilm incubated with various formulas of HCT hydrogel for 1 h under 90-degree tilt, and then subjected to 20 J·cm−2 of light illumination. Each value is the mean from three independent experiments ± standard deviation. * p < 0.05.
Antimicrobial PDI has provided new hope for controlling microbial infections. However, the area of infection might not be a plane or horizontal region. In this regards, to retain or prolong the drug delivery system on the absorption site becomes a key issue for a successful treatment. Incorporation of HPMC into HCT hydrogel can increase its adhesiveness and further warrant its therapeutic efficacy.
2.5. PDI Treatment Adjustment of HCT Hydrogel
Previously, we have shown that a 30-min incubation time with chitosan following PDI can potentiate the bactericidal efficacy in planktonic cells and biofilms [
6]. This suggests that the potentiated effect of chitosan worked after the bacterial damage induced by PDI. Therefore, the PDI efficacy of the F-1 and F-2 HCT hydrogel was examined with an additional incubation after light irradiation. As shown in
Figure 4A, after light irradiation, the complete inactivation against
S. aureus biofilm was observed with an additional 1- or 2-h incubation in the group treated with F-1 and F-2 HCT hydrogel. Although a complete eradication was not found in
P.
aeruginosa biofilm, additional incubation after PDI could result in a significant bactericidal efficacy (
Figure 4B).
In this study, after light irradiation, a longer retention time of HTC hydrogels on biofilms is required to enhance the bactericidal efficacy against S. aureus and P. aeruginosa. Meanwhile, the augmented bactericidal effect of chitosan in HTC hydrogel reduced as increasing the HPMC concentration. These results indicate that, like TBO, chitosan released from the HTC hydrogel was probably constrained due to the high viscosity network of hydrogel. Therefore, a longer retention time was required to achieve the augmented bactericidal efficacy.
Figure 4.
Cell survival fraction of S. aureus biofilm (A) and P. aeruginosa biofilm (B) after being incubated with various formulas of HCT hydrogel for 1 h and then subjected to 20 J·cm−2 of the red light illumination. After PDI, biofilm cell was maintained in HCT hydrogel for 0, 30 min, 1 h and 2 h and then plate count. Each value is the mean from three independent experiments ± standard deviation. * p < 0.05. x means no biofilm colonies were counted in this study.
Figure 4.
Cell survival fraction of S. aureus biofilm (A) and P. aeruginosa biofilm (B) after being incubated with various formulas of HCT hydrogel for 1 h and then subjected to 20 J·cm−2 of the red light illumination. After PDI, biofilm cell was maintained in HCT hydrogel for 0, 30 min, 1 h and 2 h and then plate count. Each value is the mean from three independent experiments ± standard deviation. * p < 0.05. x means no biofilm colonies were counted in this study.
2.6. In Vivo Study
To examine the
in vivo antimicrobial activity of HCT hydrogel, the PDI efficacy was verified in the animal model of rat skin burn wounds infected with
S.
aureus. These infected wounds were incubated with F-1 or F-2 HTC hydrogels for 1 h and then irradiated with 100 J·cm
−2 of 630 nm laser light.
Figure 5 demonstrates that the infected wounds treated with HCT hydrogel mediated PDI showed a significant reduction in bacterial cells survival compared to that of wounds treated with PBS. On the other hand, the overall reduction rate reduced as increasing the concentration of HPMC in HTC hydrogel. Although substantial reductions in the viable count of
S.
aureus biofilm in the wounds were achieved, the kills observed in this
in vivo model were substantially lower than those reported in
in vitro studies. Except for the biofilm formation, the reduced efficacy in the
in vivo study might be due to the host tissue competing with the photosensitizer and chitosan released from hydrogel. In this regard, tissue damage might be induced. Based on the visualized observation, we did not find significant difference on the healing of rat tissues between the groups treated with PBS or HCT hydrogel. In the future, further pathological analysis is required to examine whether HCT hydrogel will cause tissue damage after light irradiation.
Figure 5.
S. aureus viable counts in burn wounds incubated with PBS, F-1 or F-2 HCT hydrogels. After 1 h incubation, light dose of 100 J·cm−2 were applied to the burn wounds. After PDI, HCT hydrogel was maintained in the wounds for another 2 h incubation and then plate count. Reported values are the means values ± SD. * p < 0.05.
Figure 5.
S. aureus viable counts in burn wounds incubated with PBS, F-1 or F-2 HCT hydrogels. After 1 h incubation, light dose of 100 J·cm−2 were applied to the burn wounds. After PDI, HCT hydrogel was maintained in the wounds for another 2 h incubation and then plate count. Reported values are the means values ± SD. * p < 0.05.