Ultra-Fast Glyco-Coating of Non-Biological Surfaces
Abstract
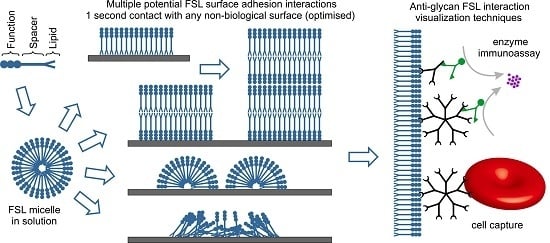
:1. Introduction

2. Results and Discussion
2.1. Surface Variations
2.1.1. Surface Variations—Coupons
| Surface | Alphabetical Listing of Materials Modified by Blood Group A Fsl Constructs |
|---|---|
| Metals | Aluminum, Copper, Gold, Nickel, silver, Stainless Steel (304), Stainless Steel (316L), Stainless Steel (347), Titanium |
| Plastics/Polymers/Rubbers/Fibres (Alphabetical Order) | Acrylonitrile butadiene styrene (ABS), Cellulose acetate (transparency film), Cellulose acetate (nanofibres), Chlorinated polyvinyl chloride (CPVC), Chlorosulfonated polyethylene (CSPE, hypalon), Cotton, Ethylene propylene diene monomer (EPDM) rubber, Mixed cellulose esters, Natural rubber, Nitrile butadiene (NBR) rubber, Nitrocellulose, Poly(methyl methacrylate) (PMMA: Plexiglass), Polyamide (Nylon), Polyamide PA66 nanofibres, Polycarbonate, Polyetheretherketone (PEEK: Arlon 1330), Polyethylene terephthalate (PET: Polyester, Dacron), Polyethylene terephthalate glycol (PETG), Polyethylene UMHW, Polypropylene, Polystyrene, Polytetrafluoroethylene (PTFE), Polyurethane (high temperature polymer), Polyvinyl butyral nanofibres (PVB), Polyvinyl chloride (PVC), Polyvinylidene fluoride (PVDF), Regenerated cellulose, Silicone rubber, Silk, Silica gel S60 (TLC plate), Silica gel C18 (TLC plate), Viton rubber, wood (various) |
| Other | Borosilicate glass, Concrete, Ceramic tile-glazed, Hydroxyapatite, Ceramic porcelain, Paper-24 varieties of coated and uncoated papers |

2.1.2. Surface Variations—Printing and Direct Application


2.1.3. Cell Adhesion to Printed FSL Constructs

2.1.4. Microsphere Binding

2.1.5. Nanofibre Incorporation

2.2. Binding Characteristics and Performance
2.2.1. Molarity

2.2.2. pH

2.2.3. Ionic Concentration

2.2.4. Effect of Surface and FSL Delivery Solutions

| Solvent | Atri-Ad-DOPE | Atri-CMG-DOPE | Atetra-CMG-DOPE | |||
|---|---|---|---|---|---|---|
| Methanol | Ethanol | Methanol | Ethanol | Methanol | Ethanol | |
| 96% | + 1 | + | + | P 2 | + | P |
| 80% | + | + | + | + | + | + |
| 70% | + | + | + | + | + | + |
2.3. Stability
2.3.1. Serum

2.3.2. Alcohol


2.3.3. Detergent/Surfactant

3. Experimental Section
3.1. Synthesis of FSL Constructs
3.2. Surfaces
3.2.1. Disc Coupons
3.2.2. Membranes and Planar Surfaces
3.2.3. Electrospinning Nanofibres Incorporating FSL Constructs
3.3. Methods for FSL Coating Surfaces
3.3.1. Direct Application
3.3.2. Inkjet Printing
3.4. Microplate Assembly
3.5. Visualisation of Blood Group A FSL Constructs
3.5.1. Enzyme Immunoassay (EIA)
3.5.2. Cell Binding via Antibody Bound to FSL
3.6. Scanning Electron Microscopy (SEM)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gautam, S.; Korchagina, E.Y.; Bovin, N.V.; Federspiel, W.J. Specific antibody filter (SAF) binding capacity enhancement to remove anti-A antibodies. J. Biomed. Mater. Res. Part B 2010, 95, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Pochechueva, T.; Jacob, F.; Goldstein, D.R.; Huflejt, M.E.; Chinarev, A.; Caduff, R.; Fink, D.; Hacker, N.; Bovin, N.V.; Heinzelmann-Schwarz, V. Comparison of suspension array, ELISA and printed glycan array in the detection of human anti-glycan antibodies. Glycoconj. J. 2011, 28, 507–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.X.; Fany, Y.; Xu, Z.K. Glycosylated membranes: A promising biomimetic material. J. Appl. Polym. Sci. 2014, 131, 39658. [Google Scholar] [CrossRef]
- Korchagina, E.Y.; Henry, S.M. Synthetic Glycolipid-like constructs as tools for glycobiology research, diagnostics and as potential therapeutics. Biochemistry (Moscow) 2015, 80, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Korchagina, E.; Tuzikov, A.; Formanovsky, A.; Popova, I.; Henry, S.; Bovin, N. Toward creating cell membrane glycolandscapes with glycan lipid constructs. Carbohydr. Res. 2012, 356, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Ilyushina, N.A.; Chernyy, E.S.; Korchagina, E.Y.; Gambaryan, A.S.; Henry, S.M.; Bovin, N.V. Labeling of influenza viruses with synthetic fluorescent and biotin-labeled lipids. Virol. Sin. 2014, 29, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.A.; Bovin, N.V.; Bess, D.; Henry, S.M. FSL Constructs: A simple method for modifying cell/virion surfaces with a range of biological markers without affecting their viability. J. Vis. Exp. 2011, 54, e3289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, K.; Korchagina, E.; Ryzhov, I.; Bovin, N.; Henry, S. Mapping the fine specificity of ABO monoclonal reagents with A and B type-specific FSL constructs in kodecytes and inkjet printed on paper. Transfusion 2014, 54, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Barr, K.; Korchagina, E.; Popova, I.; Bovin, N.; Henry, S. Monoclonal anti-A activity against the FORS1 (Forssman) antigen. Transfusion 2015, 55, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Henry, S. Magnetic Bead Coatings: Today and Tomorrow. Chapter 5 Rapid Biofunctionalization of Magnetic Beads with Function-Spacer-Lipid Constructs; SepMag: Barcelona, Spain, 2015; pp. 11–13. Available online: http://sepmag.eu/free-guide-magnetic-bead-coatings (accessed on 1 December 2015).
- Barr, K.; Kannan, B.; Korchagina, E.; Popova, I.; Ryzhov, I.; Henry, S.; Bovin, N. Biofunctionalizing nanofibres with carbohydrate blood group antigens. Biopolymers 2015. submitted for publication. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, E.; Barr, K.; Korchagina, E.; Tuzikov, A.; Henry, S.; Bovin, N. Ultra-Fast Glyco-Coating of Non-Biological Surfaces. Int. J. Mol. Sci. 2016, 17, 118. https://doi.org/10.3390/ijms17010118
Williams E, Barr K, Korchagina E, Tuzikov A, Henry S, Bovin N. Ultra-Fast Glyco-Coating of Non-Biological Surfaces. International Journal of Molecular Sciences. 2016; 17(1):118. https://doi.org/10.3390/ijms17010118
Chicago/Turabian StyleWilliams, Eleanor, Katie Barr, Elena Korchagina, Alexander Tuzikov, Stephen Henry, and Nicolai Bovin. 2016. "Ultra-Fast Glyco-Coating of Non-Biological Surfaces" International Journal of Molecular Sciences 17, no. 1: 118. https://doi.org/10.3390/ijms17010118
APA StyleWilliams, E., Barr, K., Korchagina, E., Tuzikov, A., Henry, S., & Bovin, N. (2016). Ultra-Fast Glyco-Coating of Non-Biological Surfaces. International Journal of Molecular Sciences, 17(1), 118. https://doi.org/10.3390/ijms17010118