Preparation of Low Molecular Weight Chondroitin Sulfates, Screening of a High Anti-Complement Capacity of Low Molecular Weight Chondroitin Sulfate and Its Biological Activity Studies in Attenuating Osteoarthritis
Abstract
:1. Introduction
2. Results
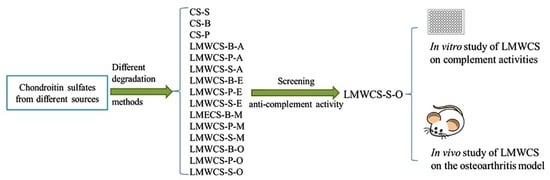
2.1. Preparation and Characterization of Low Molecular Weight Chondroitin Sulfate (LMWCSs)
2.2. In Vitro Effect of LMWCSs on Complement Activities
2.2.1. Hemolytic Analysis
2.2.2. Anti-C3 Deposition Capacity
2.2.3. Cell Viability Analysis
2.3. In Vivo Effect of LMWCSs on the OA Model
2.3.1. Functional Wind-up Determinations
2.3.2. Histological Analysis
2.3.3. Levels of C5b-9 in Serum
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Animals
4.3. Sample Preparation
4.4. Characterization of LMWCS
4.4.1. Disaccharide Composition
4.4.2. Ultraviolet–Visible (UV) Absorption
4.4.3. Nuclear Magnetic Resonance (NMR)
4.4.4. Molecular Weight Determination
4.5. In Vitro Anti-Complement Activity Analysis
4.5.1. Hemolytic Analysis
4.5.2. Anti-C3 Deposition Capacity Determination
4.5.3. Cell Viability Analysis
4.6. In Vivo Experiments
4.6.1. Animal Model and Treatment
4.6.2. Functional Wind-up Determination
4.6.3. C5b-9 Level Determinations and Histological Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Creamer, P.; Hochberg, M.C. Osteoarthritis. Lancet 1997, 350, 503–509. [Google Scholar] [CrossRef]
- Haseeb, A.; Haqqi, T.M. Immunopathogenesis of osteoarthritis. Clin. Immunol. 2013, 146, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Rozelle, A.L.; Lepus, C.M.; Scanzello, C.R.; Song, J.J.; Larsen, D.M.; Crish, J.F.; Bebek, G.; Ritter, S.Y.; Lindstrom, T.M.; et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 2011, 17, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Gadjeva, M. The complement system overview. Methods Mol. Biol. 2014, 1100, 1–9. [Google Scholar] [PubMed]
- Robinson, W.H.; Holers, V.M.; Rozelle, A.L. Complement Inhibitory Agents as Therapeutics in Posttraumatic and Degenerative Arthritis. Patent WO 2009/151634 A1, 31 December 2009. [Google Scholar]
- Li, L.; Li, Y.; Ijaz, M.; Shahbaz, M.; Lian, Q.; Wang, F. Review on complement analysis method and the roles of glycosaminoglycans in the complement system. Carbohydr. Polym. 2015, 134, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Ecker, E.E.; Gross, P. Anticomplementary power of heparin. J. Infect. Dis. 1929, 44, 250–253. [Google Scholar] [CrossRef]
- Kelly, U.; Yu, L.; Kumar, P.; Ding, J.D.; Jiang, H.; Hageman, G.S.; Arshavsky, V.Y.; Frank, M.M.; Hauser, M.A.; Rickman, C.B. Heparan sulfate, including that in Bruch’s membrane, inhibits the complement alternative pathway: Implications for age-related macular degeneration. J. Immunol. 2010, 185, 5486–5494. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Mathy, M.; Sanchez, C.; Lambert, C. Chondroitin sulfate in the treatment of osteoarthritis: From in vitro studies to clinical recommendations. Ther. Adv. Musculoskelet. Dis. 2010, 2, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Skliris, A.; Happonen, K.E.; Terpos, E.; Labropoulou, V.; Børset, M.; Heinegård, D.; Blom, A.M.; Theocharis, A.D. Serglycin inhibits the classical and lectin pathways of complement via its glycosaminoglycan chains: Implications for multiple myeloma. Eur. J. Immunol. 2011, 41, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Li, L.; Wang, F.; Yi, Q.; Dong, Q.; Sun, C.; Wang, J. A method for identifying the origin of chondroitin sulfate with near infrared spectroscopy. J. Pharm. Biomed. Anal. 2012, 61, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Lamari, F.N.; Karamanos, N.K. Structure of Chondroitin Sulfate. In Advances in Pharmacology; Academic Press: Cambridge, MA, USA, 2006; pp. 33–48. [Google Scholar]
- Lauder, R.M. Chondroitin sulphate: A complex molecule with potential impacts on a wide range of biological systems. Complement. Ther. Med. 2009, 17, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Silva, L. Isolation and purification of chondroitin sulfate. Adv. Pharmacol. 2006, 53, 21–31. [Google Scholar] [PubMed]
- Volpi, N. Disaccharide mapping of chondroitin sulfate of different origins by high-performance capillary electrophoresis and high-performance liquid chromatography. Carbohydr. Polym. 2004, 55, 273–281. [Google Scholar] [CrossRef]
- Baici, A.; Hörler, D.; Moser, B.; Hofer, H.; Fehr, K.; Wagenhäuser, F. Analysis of glycosaminoglycans in human serum after oral administration of chondroitin sulfate. Rheumatol. Int. 1992, 12, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, P.; Cheng, Y.; Zhang, X.; Sheng, J.; Wang, D.; Li, J.; Zhang, Q.; Zhong, C.; Cao, R.; et al. Enhancing the intestinal absorption of low molecular weight chondroitin sulfate by conjugation with α-linolenic acid and the transport mechanism of the conjugates. Int. J. Pharm. 2014, 465, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Sim, J.S.; Jeong, C.S.; Chang, S.Y.; Choi, D.W.; Toida, T.; Kim, Y.S. Effects of low molecular weight chondroitin sulfate on type II collagen-induced arthritis in DBA/1J mice. Biol. Pharm. Bull. 2004, 27, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Kogelberg, H.; Lawson, A.M. Generation and structural characterization of a range of unmodified chondroitin sulfate oligosaccharide fragments. Anal. Biochem. 1996, 237, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Wu, M.; Liu, S.; Lian, W.; Li, Z.; Zhao, J. Preparation and characterization of O-acylated Fucosylated chondroitin sulfate from sea cucumber. Mar. Drugs 2012, 10, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Bexborn, F.; Andersson, P.O.; Chen, H.; Nilsson, B.; Ekdahl, K.N. The tick-over theory revisited: Formation and regulation of the soluble alternative complement C3 convertase (C3(H2O)Bb). Mol. Immunol. 2008, 45, 2370–2379. [Google Scholar] [CrossRef] [PubMed]
- Lemare, F.; Steimberg, N.; Le Griel, C.; Demignot, S.; Adolphe, M. Dedifferentiated chondrocytes cultured in alginate beads: Restoration of the differentiated phenotype and of the metabolic responses to interleukin-1beta. J. Cell. Physiol. 1998, 176, 303–313. [Google Scholar] [CrossRef]
- Sommaggio, R.; Perez-Cruz, M.; Brokaw, J.L.; Manez, R.; Costa, C. Inhibition of complement component C5 protects porcine chondrocytes from xenogeneic rejection. Osteoarthr. Cartil. 2013, 21, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Beier, F. Mouse models of osteoarthritis: Modelling risk factors and assessing outcomes. Nat. Rev. Rheumatol. 2014, 10, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S. In vivo osteoarthritis target validation utilizing genetically-modified mice. Curr. Drug Targets 2007, 8, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Harden, R.N.; Wallach, G.; Gagnon, C.M.; Zereshki, A.; Mukai, A.; Saracoglu, M.; Kuroda, M.M.; Graciosa, J.R.; Bruehl, S. The osteoarthritis knee model: Psychophysical characteristics and putative outcomes. J. Pain 2013, 14, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, B.; Liu, C.J. Establishment of a surgically-induced model in mice to investigate the protective role of progranulin in osteoarthritis. J. Vis. Exp. 2014, 84, e50924. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Ronca, F.; Palmieri, L.; Panicucci, P.; Ronca, G. Anti-inflammatory activity of chondroitin sulfate. Osteoarthr. Cartil. 1998, 6, 14–21. [Google Scholar] [CrossRef]
- Du Souich, P. Absorption, distribution and mechanism of action of SYSADOAS. Pharmacol. Ther. 2014, 142, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Farran, A.; Montell, E.; Verges, J.; Pelletier, J.P. Discrepancies in composition and biological effects of different formulations of chondroitin sulfate. Molecules 2015, 20, 4277–4289. [Google Scholar] [CrossRef] [PubMed]
- Servais, G.; Walmagh, J.; Duchateau, J. Simple quantitative haemolytic microassay for determination of complement alternative pathway activation (AP50). J. Immunol. Methods 1991, 140, 93–100. [Google Scholar] [CrossRef]
- Banda, N.K.; Takahashi, K. Analysis of the complement activation in mice. In The Complement System; Springer: Berlin/Heidelberg, Germany, 2014; pp. 365–371. [Google Scholar]
- Gosset, M.; Berenbaum, F.; Thirion, S.; Jacques, C. Primary culture and phenotyping of murine chondrocytes. Nat. Protoc. 2008, 3, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Hoff, J. Methods of blood collection in the mouse. Lab. Anim. 2000, 29, 47–53. [Google Scholar]









| Sample Name | ΔDi-0S (%) | ΔDi-6S (%) | ΔDi-4S (%) | ΔDi-2,6diS (%) | ΔDi-4,6diS (%) | (Da) |
|---|---|---|---|---|---|---|
| LMWCS-B-A | 25.16 | 26.16 | 48.07 | – | – | 1826 |
| LMWCS-P-A | 30.89 | 27.24 | 41.25 | – | – | 2690 |
| LMWCS-S-A | 23.34 | 37.25 | 28.19 | 9.30 | 1.92 | 1217 |
| LMWCS-B-E | 3.78 | 16.50 | 78.94 | – | – | 2583 |
| LMWCS-P-E | 2.48 | 46.88 | 49.18 | – | – | 2920 |
| LMWCS-S-E | 0.00 | 60.87 | 24.38 | 9.36 | 5.39 | 1994 |
| LMWCS-B-M | 35.83 | 22.42 | 41.22 | – | – | 8085 |
| LMWCS-P-M | 16.01 | 29.94 | 53.37 | – | – | 3662 |
| LMWCS-S-M | 43.97 | 29.74 | 18.36 | 7.52 | 0.41 | 4269 |
| LMWCS-B-O | 4.86 | 25.77 | 68.78 | – | – | 1561 |
| LMWCS-P-O | 4.26 | 26.87 | 68.60 | – | – | 2191 |
| LMWCS-S-O | 9.22 | 50.02 | 27.50 | 12.17 | 1.08 | 1511 |
| CS-B | 7.30 | 23.54 | 68.82 | – | – | 18,150 |
| CS-P | 0.88 | 32.12 | 66.68 | – | – | 10,070 |
| CS-S | 0.00 | 54.77 | 31.31 | 12.66 | 1.26 | 31,300 |
| Sample | Line Equation | IC50 (mg) |
|---|---|---|
| LMWCS-B-A | y = 0.0967x + 0.7583 R = 0.989 | 5.59 |
| LMWCS-P-A | y = 0.11x + 1.8179 R = 0.989 | 7.32 |
| LMWCS-S-A | y = 0.0703x + 1.2167 R = 0.9599 | 4.76 |
| LMWCS-B-E | y = 0.0703x + 0.8147 R = 0.980 | 4.33 |
| LMWCS-P-E | y = 0.064x + 1.8861 R = 0.992 | 5.09 |
| LMWCS-S-E | y = 0.0589x + 1.2021 R = 0.977 | 4.15 |
| LMWCS-B-M | y = 0.1016x − 1.3729 R = 0.984 | 3.71 |
| LMWCS-P-M | y = 0.0613x + 1.5563 R = 0.994 | 4.62 |
| LMWCS-S-M | y = 0.0815x + 1.1212 R = 0.991 | 5.20 |
| LMWCS-B-O | y = 0.0891x + 0.3776 R = 0.994 | 4.83 |
| LMWCS-P-O | y = 0.0573x + 1.425 R = 0.975 | 4.29 |
| LMWCS-S-O | y = 0.0565x + 0.5873 R = 0.988 | 3.41 |
| CS-B | y = 0.1029x + 1.0596 R = 0.993 | 6.20 |
| CS-P | y = 0.1499x + 1.5709 R = 0.988 | 9.07 |
| CS-S | y = 0.0902x − 0.0251 R = 0.987 | 4.48 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, Y.; Feng, D.; Xu, L.; Yin, F.; Zang, H.; Liu, C.; Wang, F. Preparation of Low Molecular Weight Chondroitin Sulfates, Screening of a High Anti-Complement Capacity of Low Molecular Weight Chondroitin Sulfate and Its Biological Activity Studies in Attenuating Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 1685. https://doi.org/10.3390/ijms17101685
Li L, Li Y, Feng D, Xu L, Yin F, Zang H, Liu C, Wang F. Preparation of Low Molecular Weight Chondroitin Sulfates, Screening of a High Anti-Complement Capacity of Low Molecular Weight Chondroitin Sulfate and Its Biological Activity Studies in Attenuating Osteoarthritis. International Journal of Molecular Sciences. 2016; 17(10):1685. https://doi.org/10.3390/ijms17101685
Chicago/Turabian StyleLi, Lian, Yan Li, Danyang Feng, Linghua Xu, Fengxin Yin, Hengchang Zang, Chunhui Liu, and Fengshan Wang. 2016. "Preparation of Low Molecular Weight Chondroitin Sulfates, Screening of a High Anti-Complement Capacity of Low Molecular Weight Chondroitin Sulfate and Its Biological Activity Studies in Attenuating Osteoarthritis" International Journal of Molecular Sciences 17, no. 10: 1685. https://doi.org/10.3390/ijms17101685
APA StyleLi, L., Li, Y., Feng, D., Xu, L., Yin, F., Zang, H., Liu, C., & Wang, F. (2016). Preparation of Low Molecular Weight Chondroitin Sulfates, Screening of a High Anti-Complement Capacity of Low Molecular Weight Chondroitin Sulfate and Its Biological Activity Studies in Attenuating Osteoarthritis. International Journal of Molecular Sciences, 17(10), 1685. https://doi.org/10.3390/ijms17101685