Early Antipsychotic Treatment in Juvenile Rats Elicits Long-Term Alterations to the Dopamine Neurotransmitter System
Abstract
:1. Introduction
2. Results
2.1. Long-Term Effects of Adolescent APD Treatment on Tyrosine Hydroxylase (TH) and Phosphorylated-Tyrosine Hydroxylase (p-TH) Levels
2.1.1. Tyrosine Hydroxylase
2.1.2. Phosphorylated-Tyrosine Hydroxylase
2.2. Long-Term Effects of Adolescent APD Treatment on Dopamine Active Transporter (DAT) Levels
2.3. Long-Term Effects of Adolescent APD Treatment on Dopamine D1 Receptor (D1R) Levels
2.4. Long-Term Effects of Adolescent APD Treatment on Dopamine D2 Receptor (D2R) Levels
3. Discussion
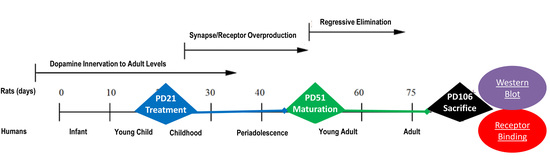
4. Materials and Methods
4.1. Animals and Housing
4.2. Drug Treatment Groups
4.3. Histological Procedures
4.3.1. Histology—Microdissection (Western Blot Analyses)
4.3.2. Histology—Receptor Autoradiography
4.4. Western Blot Analyses
4.5. Receptor Autoradiography and Quantification
4.5.1. Dopamine Active Transporter (DAT) Binding Procedures
4.5.2. Dopamine D1 Receptor Binding Procedures
4.5.3. Dopamine D2 Receptor Binding Procedures
4.5.4. Quantification
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| ANOVA | Analysis of Variance |
| APD | Antipsychotic Drug |
| CPu | Caudate Putamen |
| DA | Dopamine |
| DAT | Dopamine Active Transporter |
| DOPA | 3,4-Dihydroxyphenylalanine |
| HED | Human Equivalent Dose |
| HRP | Horseradish Peroxidase |
| NAc | Nucleus Accumbens |
| NT | Neurotransmitter |
| p-TH | Phosphorylated-Tyrosine Hydroxylase |
| PD | Postnatal Day |
| PFC | Prefrontal Cortex |
| PVDF | Polyvinylidene Difluoride |
| RT | Room Temperature |
| SEM | Standard Error of the Mean |
| SN | Substantia Nigra |
| TBST | Tris-Buffered Saline-Tween |
| TH | Tyrosine Hydroxylase |
| VTA | Ventral Tegmental Area |
References
- Alexander, G.C.; Gallagher, S.A.; Mascola, A.; Moloney, R.M.; Stafford, R.S. Increasing off-label use of antipsychotic medications in the United States. Pharmacoepidemiol. Drug Saf. 2011, 20, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Seida, J.C.; Schouten, J.R.; Boylan, K.; Newton, A.S.; Mousavi, S.S.; Beaith, A.; Vandermeer, B.; Dryden, D.M.; Carrey, N. Antipsychotics for children and young adults: A comparative effectiveness review. Pediatrics 2012, 129, e771–e784. [Google Scholar] [CrossRef] [PubMed]
- Olfson, M.; Blanco, C.; Liu, S.; Wang, S.; Correll, C.U. National trends in the office-based treatment of children, adolescents, and adults with antipsychotics. Arch. Gen. Psychiatry 2012, 69, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Rani, F.; Murray, M.L.; Byrne, P.J.; Wong, I.C.K. Epidemiologic features of antipsychotic prescribing to children and adolescents in primary care in the United Kingdom. Pediatrics 2008, 121, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Olfson, M.; Crystal, S.; Huang, C.; Gerhard, T. Trends in antipsychotic drug use by very young, privately insured children. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 13–23. [Google Scholar] [PubMed]
- Varley, C.K.; McClellan, J. Implications of marked weight gain associated with atypical antipsychotic medications in children and adolescents. JAMA 2009, 302, 1811–1812. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, P.J. Risperidone for non-psychotic disorders in paediatric patients: Which child is to benefit? Dev. Med. Child Neurol. 2014, 56, 919–920. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L.; Navalta, C.P. Altering the course of neurodevelopment: A framework for understanding the enduring effects of psychotropic drugs. Int. J. Dev. Neurosci. 2004, 22, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Memarzia, J.; Tracy, D.; Giaroli, G. The use of antipsychotics in preschoolers: A veto or a sensible last option? J. Psychopharmacol. 2014, 28, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Taylor, D.; Zalsman, G.; Frangou, S.; Kyriakopoulos, M. Antipsychotics use in children and adolescents: An on-going challenge in clinical practice. J. Psychopharmacol. 2014, 28, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Haw, C.; Stubbs, J. Off-label use of antipsychotics: Are we mad? Expert Opin. Drug Saf. 2007, 6, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, B.; Correll, C.; van Zwieten-Boot, B.; Zuddas, A.; Parellada, M.; Arango, C. Antipsychotics in children and adolescents: Increasing use, evidence for efficacy and safety concerns. Eur. Neuropsychopharmacol. 2009, 19, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shaw, S.R. Efficacy of risperidone in managing maladaptive behaviors for children with autistic spectrum disorder: A meta-analysis. J. Pediatr. Health Care 2012, 26, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Pompili, M.; Baldessarini, R.J.; Forte, A.; Erbuto, D.; Serafini, G.; Fiorillo, A.; Amore, M.; Girardi, P. Do atypical antipsychotics have antisuicidal effects? A hypothesis-generating overview. Int. J. Mol. Sci. 2016, 17, 1700. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A.; Floresco, S.B.; Goto, Y.; Lodge, D.J. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007, 30, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Kegeles, L.S.; Abi-Dargham, A.; Frankle, W.G.; Gil, R.; Cooper, T.B.; Slifstein, M.; Hwang, D.-R.; Huang, Y.; Haber, S.N.; Laruelle, M. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch. Gen. Psychiatry 2010, 67, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Purves-Tyson, T.D.; Handelsman, D.J.; Double, K.L.; Owens, S.J.; Bustamante, S.; Weickert, C.S. Testosterone regulation of sex steroid-related mRNAs and dopamine-related mRNAs in adolescent male rat substantia nigra. BMC Neurosci. 2012, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Milstein, J.A.; Elnabawi, A.; Vinish, M.; Swanson, T.; Enos, J.K.; Bailey, A.M.; Kolb, B.; Frost, D.O. Olanzapine treatment of adolescent rats causes enduring specific memory impairments and alters cortical development and function. PLoS ONE 2013, 8, e57308. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y. Mechanism of Action of Atypical Antipsychotic Drugs. In Neuropsychopharmacology: The Fifth Generation of Progress; Davis, K.L., Charney, D., Coyle, J.T., Nemeroff, C., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2002; pp. 819–831. [Google Scholar]
- Nasrallah, H.A. Atypical antipsychotic-induced metabolic side effects: Insights from receptor-binding profiles. Mol. Psychiatry 2008, 13, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Amato, D. Serotonin in antipsychotic drugs action. Behav. Brain Res. 2015, 277, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Levitt, P.; Harvey, J.A.; Friedman, E.; Simansky, K.; Murphy, E.H. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997, 20, 269–274. [Google Scholar] [CrossRef]
- Frost, D.O.; Cadet, J.L. Effects of methamphetamine-induced neurotoxicity on the development of neural circuitry: A hypothesis. Behav. Brain Res. 2000, 34, 103–118. [Google Scholar] [CrossRef]
- Klomp, A.; Tremoleda, J.L.; Wylezinska, M.; Nederveen, A.J.; Feenstra, M.; Gsell, W.; Reneman, L. Lasting effects of chronic fluoxetine treatment on the late developing rat brain: Age-dependent changes in the serotonergic neurotransmitter system assessed by pharmacological MRI. NeuroImage 2012, 59, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003, 27, 3–18. [Google Scholar] [CrossRef]
- Marco, E.M.; Adriani, W.; Ruocco, L.A.; Canese, R.; Sadile, A.G.; Laviola, G. Neurobehavioral adaptations to methylphenidate: The issue of early adolescent exposure. Neurosci. Biobehav. Rev. 2011, 35, 1722–1739. [Google Scholar] [CrossRef] [PubMed]
- Cousins, L.; Goodyer, I.M. Antidepressants and the adolescent brain. J. Psychopharmacol. 2015, 29, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Bottelier, M.A.; Schouw, M.L.J.; Klomp, A.; Tamminga, H.G.H.; Schrantee, A.G.M.; Bouziane, C.; de Ruiter, M.B.; Boer, F.; Ruhé, H.G.; Denys, D.; et al. The effects of psychotropic drugs on developing brain (ePOD) study: Methods and design. BMC Psychiatry 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U. From receptor pharmacology to improved outcomes: Individualising the selection, dosing, and switching of antipsychotics. Eur. Psychiatry 2010, 25, S12–S21. [Google Scholar] [CrossRef]
- Kesby, J.P.; Cui, X.; Burne, T.H.J.; Eyles, D.W. Altered dopamine ontogeny in the developmentally vitamin D deficient rat and its relevance to schizophrenia. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.; Lian, J.; Huang, X.F.; Deng, C. Early antipsychotic treatment in childhood/adolescent period has long-term effects on depressive-like, anxiety-like and locomotor behaviours in adult rats. J. Psychopharmacol. 2016, 30, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Kumra, S.; Oberstar, J.V.; Sikich, L.; Findling, R.L.; McClellan, J.M.; Vinogradov, S.; Schulz, S.C. Efficacy and tolerability of second-generation antipsychotics in children and adolescents with schizophrenia. Schizophr. Bull. 2008, 34, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Stigler, K.A.; McDougle, C.J.; Posey, D.J.; Potenza, M.N. Weight gain associated with atypical antipsychotic use in children and adolescents: Prevalence, clinical relevance, and management. Pediatr. Drugs 2004, 6, 33–44. [Google Scholar] [CrossRef]
- Zuddas, A.; Zanni, R.; Usala, T. Second generation antipsychotics (SGAs) for non-psychotic disorders in children and adolescents: A review of the randomized controlled studies. Eur. Neuropsychopharmacol. 2011, 21, 600–620. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Hu, G.; Li, M. Adult response to olanzapine or clozapine treatment is altered by adolescent antipsychotic exposure: A preclinical test in the phencyclidine hyperlocomotion model. J. Psychopharmacol. 2014, 28, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Varela, F.A.; Der-Ghazarian, T.; Lee, R.J.; Charntikov, S.; Crawford, C.A.; McDougall, S.A. Repeated aripiprazole treatment causes dopamine D2 receptor up-regulation and dopamine supersensitivity in young rats. J. Psychopharmacol. 2014, 28, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Vinish, M.; Elnabawi, A.; Milstein, J.A.; Burke, J.S.; Kallevang, J.K.; Turek, K.C.; Lansink, C.S.; Merchenthaler, I.; Bailey, A.M.; Kolb, B.; et al. Olanzapine treatment of adolescent rats alters adult reward behavior and nucleus accumbens function. Int. J. Neuropsychopharmacol. 2012, 16, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Moran-Gates, T.; Gan, L.; Park, Y.S.; Zhang, K.; Baldessarini, R.J.; Tarazi, F.I. Repeated antipsychotic drug exposurein developing rats: Dopamine receptor effects. Synapse 2006, 59, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Maciag, D.; Simpson, K.L.; Coppinger, D.; Lu, Y.; Wang, Y.; Lin, R.C.S.; Paul, I.A. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology 2006, 31, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Pan, B.; Deng, C. Early antipsychotic exposure affects serotonin and dopamine receptor binding density differently in selected brain loci of male and female juvenile rats. Pharmacol. Rep. 2016, 68, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.O.; Page, S.C.; Carroll, C.; Kolb, B. Early exposure to haloperidol or olanzapine induces long-term alterations of dendritic form. Synapse 2010, 64, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier: London, UK, 2007. [Google Scholar]
- Der-Ghazarian, T.; Charntikov, S.; Varela, F.; Crawford, C.; McDougall, S. Effects of repeated and acute aripiprazole or haloperidol treatment on dopamine synthesis in the dorsal striatum of young rats: Comparison to adult rats. J. Neural Transm. 2010, 117, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; Koprivica, V.; Dunn, R.; Tottori, K.; Kikuchi, T.; Altar, C.A. In vivo effects of aripiprazole on cortical and striatal dopaminergic and serotonergic function. Eur. J. Pharmacol. 2004, 483, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ichikawa, J.; Dai, J.; Meltzer, H.Y. Aripiprazole, a novel antipsychotic drug, preferentially increases dopamine release in the prefrontal cortex and hippocampus in rat brain. Eur. J. Pharmacol. 2004, 493, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, A.; Fabbri, D.; Heidbreder, C.A. Aripiprazole increases dopamine but not noradrenaline and serotonin levels in the mouse prefrontal cortex. Neurosci. Lett. 2005, 387, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, S.; Yamamura, S.; Nakagawa, M.; Motomura, E.; Okada, M. Dopamine D2 and serotonin 5-H.T.1A receptors mediate the actions of aripiprazole in mesocortical and mesoaccumbens transmission. Neuropharmacology 2012, 62, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Etievant, A.; Betry, C.; Arnt, J.; Haddjeri, N. Bifeprunox and aripiprazole suppress in vivo VTA dopaminergic neuronal activity via D2 and not D3 dopamine autoreceptor activation. Neurosci. Lett. 2009, 460, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Lim, E.P.; Han, S.P.; Nagarajah, R.; Dawe, G.S. Chronic high-dose haloperidol has qualitatively similar effects to risperidone and clozapine on immediate-early gene and tyrosine hydroxylase expression in the rat locus coeruleus but not medial prefrontal cortex. Neurosci. Res. 2007, 57, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Dahan, L.; Husum, H.; Mnie-Filali, O.; Arnt, J.; Hertel, P.; Haddjeri, N. Effects of bifeprunox and aripiprazole on rat serotonin and dopamine neuronal activity and anxiolytic behaviour. J. Psychopharmacol. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Tanda, G.; Valentini, V.; de Luca, M.A.; Perra, V.; Serra, G.P.; Di Chiara, G. A systematic microdialysis study of dopamine transmission in the accumbens shell/core and prefrontal cortex after acute antipsychotics. Psychopharmacology 2015, 232, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.L.; Bridges, S.R.; Weirs, W.J. Alteration of dopamine transport in the striatum and nucleus accumbens of ovariectomized and estrogen-primed rats following N-(p-isothiocyanatophenethyl) spiperone (NIPS) treatment. Brain Res. Bull. 2001, 54, 631–638. [Google Scholar] [CrossRef]
- Gulley, J.M.; Zahniser, N.R. Rapid regulation of dopamine transporter function by substrates, blockers and presynaptic receptor ligands. Eur. J. Pharmacol. 2003, 479, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, H.L.; Joyce, A.R.; Carroll, F.I.; Kuhar, M.J. Dopamine D1 and D2 receptors influence dopamine transporter synthesis and degradation in the rat. J. Pharmacol. Exp. Ther. 2001, 298, 129–140. [Google Scholar] [PubMed]
- Sinclair, D.; Purves-Tyson, T.D.; Allen, K.M.; Weickert, C.S. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology 2014, 231, 1581–1599. [Google Scholar] [CrossRef] [PubMed]
- Borgkvist, A.; Malmlöf, T.; Feltmann, K.; Lindskog, M.; Schilström, B. Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int. J. Neuropsychopharmacol. 2012, 15, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Tarazi, F.I.; Zhang, K.; Baldessarini, R.J. Long-term effects of olanzapine, risperidone, and quetiapine on dopamine receptor types in regions of rat brain: Implications for antipsychotic drug treatment. J. Pharmacol. Exp. Ther. 2001, 297, 711–717. [Google Scholar] [PubMed]
- Grace, A.A. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res. Rev. 2000, 31, 330–341. [Google Scholar] [CrossRef]
- Goto, Y.; Grace, A.A. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat. Neurosci. 2005, 8, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P. Dopamine D2High receptors moderately elevated by bifeprunox and aripiprazole. Synapse 2008, 62, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, S.; Okamura, N.; Sekine, Y.; Kanahara, N.; Hashimoto, K.; Iyo, M. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr. Bull. 2011. [Google Scholar] [CrossRef] [PubMed]
- Understanding the Risks of Antipsychotic Treatment in Young People. Harv. Ment. Health Lett. 2010. Available online: http://www.health.harvard.edu/newsletter_article/Understanding_the_risks_of_antipsychotic_treatment_in_young_people (accessed on 18 November 2016).
- Griffon, N.; Sokoloff, P.; Diaz, J.; Lévesque, D.; Sautel, F.; Schwartz, J.C.; Simon, P.; Costentin, J.; Garrido, F.; Mann, A.; et al. The dopamine D3 receptor and schizophrenia: Pharmacological, anatomical and genetic approaches. Eur. Neuropsychopharmacol. 1995, 5, 3–9. [Google Scholar] [CrossRef]
- Constantine, R.J.; Boaz, T.; Tandon, R. Antipsychotic polypharmacy in the treatment of children and adolescents in the fee-for-service component of a large state medicaid program. Clin. Ther. 2010, 32, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Purves-Tyson, T.D.; Owens, S.J.; Double, K.L.; Desai, R.; Handelsman, D.J.; Weickert, C.S. Testosterone induces molecular changes in dopamine signaling pathway molecules in the adolescent male rat nigrostriatal pathway. PLoS ONE 2014, 9, e91151. [Google Scholar] [CrossRef] [PubMed]
- Gogos, A.; Kwek, P.; van den Buuse, M. The role of estrogen and testosterone in female rats in behavioral models of relevance to schizophrenia. Psychopharmacology 2012, 219, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.W.; Nemeroff, C.B. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 2007, 64, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.; Paton, C.; Kapur, S. The Maudsley Prescribing Guidelines, 10th ed.; Informa Healthcare: London, UK, 2009. [Google Scholar]
- Aravagiri, M.; Marder, S.R. Brain, plasma and tissue pharmacokinetics of risperidone and 9-hydroxyrisperidone after separate oral administration to rats. Psychopharmacology 2002, 159, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; U.S. Center for Drug Evaluation and Research, Department of Health and Human Services: Washington, WA, USA, 2005.
- Wadenberg, M.L.G. Bifeprunox: A novel antipsychotic agent with partial agonist properties at dopamine D2 and serotonin 5-HT1A receptors. Future Neurol. 2007, 2, 153–165. [Google Scholar] [CrossRef]
- Kapur, S.; VanderSpek, S.C.; Brownlee, B.A.; Nobrega, J.N. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: A suggested solution based on in vivo occupancy. J. Pharmacol. Exp. Ther. 2003, 305, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Reckless, G.E.; Nobrega, J.N.; Fletcher, P.J.; Kapur, S. Dissociation between in vivo occupancy and functional antagonism of dopamine D2 receptors: Comparing aripiprazole to other antipsychotics in animal models. Neuropsychopharmacology 2006, 31, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; De Santis, M.; He, M.; Deng, C. Risperidone-induced weight gain and reduced locomotor activity in juvenile female rats: The role of histaminergic and NPY pathways. Pharmacol. Res. 2015, 95, 20–26. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, Q.; Deng, C.; Wang, H.; Lian, J.; Huang, X.F. Hypothalamic histamine H1 receptor-AMP K. signaling time-dependently mediates olanzapine-induced hyperphagia and weight gain in female rats. Psychoneuroendocrinology 2014, 42, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lian, J.; He, M.; Deng, C.; Wang, H.; Huang, X.F. Olanzapine reduced brown adipose tissue thermogenesis and locomotor activity in female rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 51, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, M.; Deng, C.; Wang, H.; Lian, J.; Huang, X.F. Hypothalamic ghrelin signalling mediates olanzapine-induced hyperphagia and weight gain in female rats. Int. J. Neuropsychopharmacol. 2014, 17, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Pan, B.; Hu, C.H.; Han, M.; Huang, X.F. Differential effects of short- and long-term antipsychotic treatment on the expression of neuregulin-1 and ErbB4 receptors in the rat brain. Psychiatry Res. 2015, 225, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, B.; Centeno, M.; Pollandt, S.; Fu, Y.; Genzer, K.; Liu, J.; Gallagher, J.P.; Shinnick-Gallagher, P. Dopamine receptor mechanisms mediate corticotropin-releasing factor-induced long-term potentiation in the rat amygdala following cocaine withdrawal. Eur. J. Neurosci. 2010, 31, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.R.; Jotty, K.; Locke, J.L.; Jones, S.R.; Valenzuela, C.F. Moderate alcohol exposure during the rat equivalent to the third trimester of human pregnancy alters regulation of GABAA receptor-mediated synaptic transmission by dopamine in the basolateral amygdala. Front. Pediatr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Karabacak, Y.; Sase, S.; Aher, Y.D.; Sase, A.; Saroja, S.R.; Cicvaric, A.; Höger, H.; Berger, M.; Bakulev, V.; Sitte, H. The effect of modafinil on the rat dopamine transporter and dopamine receptors D1–D3 paralleling cognitive enhancement in the radial arm maze. Front. Behav. Neurosci. 2015, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Guo, X.; Xiong, F.; Cheng, G.; Lu, Q.; Yan, H. Acrylamide increases dopamine levels by affecting dopamine transport and metabolism related genes in the striatal dopaminergic system. Toxicol. Lett. 2015, 236, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Chen, J.; Lian, J.; Huang, X.F.; Deng, C. Unique effects of acute aripiprazole treatment on the dopamine D2 receptor downstream cAMP-PKA and Akt-GSK 3β signalling pathways in rats. PLoS ONE 2015, 10, e0132722. [Google Scholar]
- Kesby, J.P.; O’Loan, J.C.; Alexander, S.; Deng, C.; Huang, X.F.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.J. Developmental vitamin D deficiency alters MK-801-induced behaviours in adult offspring. Psychopharmacology 2012, 220, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Huang, X.F.; Pai, N.; Deng, C. Chronic betahistine co-treatment reverses olanzapine’s effects on dopamine D2 but not 5-HT2A/2C bindings in rat brains. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 56, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Huang, X.F.; Deng, C. Aripiprazole differentially affects mesolimbic and nigrostriatal dopaminergic transmission: Implications for long-term drug efficacy and low extrapyramidal side-effects. Int. J. Neuropsychopharmacol. 2009, 12, 941–952. [Google Scholar] [CrossRef] [PubMed]
- du Bois, T.M.; Hsu, C.W.; Li, Y.; Tan, Y.Y.; Deng, C.; Huang, X.F. Altered dopamine receptor and dopamine transporter binding and tyrosine hydroxylase mRNA expression following perinatal, NMDA receptor blockade. Neurochem. Res. 2008, 33, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Huang, X.F.; Pai, N.; Deng, C. Effects of olanzapine and betahistine co-treatment on serotonin transporter, 5-HT2A and dopamine D2 receptor binding density. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 47, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Moe, A.A.K.; Kurniawan, N.D.; Alexander, S.; Cui, X.; Burne, T.H.J.; Eyles, D.W. Risperidone induces long-lasting changes in the conditioned avoidance response and accumbal gene expression selectively in animals treated as adolescents. Neuropharmacology 2016, 108, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Guiard, B.P.; El Mansari, M.; Blier, P. Cross-talk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus ceruleus, and dorsal hippocampus. Mol. Pharmacol. 2008, 74, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Kusljic, S.; Copolov, D.L.; van den Buuse, M. Differential role of serotonergic projections arising from the dorsal and median raphe nuclei in locomotor hyperactivity and prepulse inhibition. Neuropsychopharmacology 2003, 28, 2138–2147. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Santis, M.; Lian, J.; Huang, X.-F.; Deng, C. Early Antipsychotic Treatment in Juvenile Rats Elicits Long-Term Alterations to the Dopamine Neurotransmitter System. Int. J. Mol. Sci. 2016, 17, 1944. https://doi.org/10.3390/ijms17111944
De Santis M, Lian J, Huang X-F, Deng C. Early Antipsychotic Treatment in Juvenile Rats Elicits Long-Term Alterations to the Dopamine Neurotransmitter System. International Journal of Molecular Sciences. 2016; 17(11):1944. https://doi.org/10.3390/ijms17111944
Chicago/Turabian StyleDe Santis, Michael, Jiamei Lian, Xu-Feng Huang, and Chao Deng. 2016. "Early Antipsychotic Treatment in Juvenile Rats Elicits Long-Term Alterations to the Dopamine Neurotransmitter System" International Journal of Molecular Sciences 17, no. 11: 1944. https://doi.org/10.3390/ijms17111944
APA StyleDe Santis, M., Lian, J., Huang, X. -F., & Deng, C. (2016). Early Antipsychotic Treatment in Juvenile Rats Elicits Long-Term Alterations to the Dopamine Neurotransmitter System. International Journal of Molecular Sciences, 17(11), 1944. https://doi.org/10.3390/ijms17111944