Metabolomics and Its Application in the Development of Discovering Biomarkers for Osteoporosis Research
Abstract
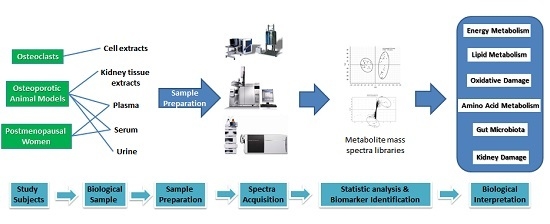
:1. Introduction
2. Study Subjects
2.1. Osteoclasts
2.2. Osteoporotic Animal Models
2.3. Postmenopausal Women
3. Sample Preparation
4. The Biomarkers and Their Potential Values in Osteoporosis Research
4.1. Potential Application in Prediction of Osteoporosis
4.2. Potential Application in Diagnosis of Osteoporosis
4.3. Potential Application in Therapeutics of Osteoporosis
4.3.1. Estrogen Derivatives
4.3.2. Bisphosphonates
4.3.3. TCM and Herbal Formula Extracts
4.3.4. Calcium, Vitamin D3 and Exercise
5. Biological and Metabolic Significance of the Biomarkers
5.1. Energy Metabolism
5.2. Lipid Metabolism and Oxidative Damage
5.3. Amino Acid Metabolism
5.4. Gut Microbiota
5.5. Kidney Damage
6. Chronological Metabolomics Data
7. Potential of Multi-Omics Integration in Osteoporosis Research
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BMD | Bone mineral density |
| CypD | Cyclophilin D |
| DSS | 2,2-dimethyl-2-silapentane-5-sulfonic acid |
| FDP-Sr | Strontium fructose 1,6-diphosphate |
| HDL-C | High-density lipoprotein cholesterol |
| 1H NMR | Proton nuclear magnetic resonance |
| HRT | Hormone replacement therapy |
| GC/MS | Gas chromatography/ mass spectrometry |
| GPCho | Glycerophosphorylcholine |
| LC/MS | Liquid chromatography/mass spectrometry |
| LDL | Low-density lipoprotein |
| LDL-C | Low-density lipoprotein cholesterol |
| LysoPC | Lysophosphatidylcholine |
| MSC | Marrow stromal stem cell |
| MS | Mass spectrometry |
| MSTFA | N-methyl-N-(trimethylsilyl) trifluoroacetamide |
| NAc | N-acetyl-l-cysteine |
| OAc | O-acetyl-l-cysteine |
| OVX | Ovariectomized |
| PBS | Sodium phosphate buffer |
| PCA | Principal component analysis |
| PLS-DA | Partial least squares-discriminant analysis |
| PPARγ | Peroxisome proliferator activated receptor γ |
| PTFE | Polytetrafluoroethylene |
| RANKL | Receptor activator of NF-κB ligand |
| ROS | Reactive oxygen species |
| SD | Spray Dawley |
| SNP | Single nucleotide polymorphisms |
| TCA | Tricarboxylic acid |
| TCM | Traditional Chinese Medicine |
| TMAO | Trimetlylamine oxide |
| TMCS | Trimethylchlorosilane |
| TSP | Tribasic sodium phosphate |
| vLDL | Very low-density lipoprotein |
References
- Wilson, I.D.; Plumb, R.; Granger, J.; Major, H.; Williams, R.; Lenz, E.M. HPLC-MS-based methods for the study of metabonomics. J. Chromatogr. B 2005, 817, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Smilde, A.K.; Westerhuis, J.A.; Hoefsloot, H.C.; Bijlsma, S.; Rubingh, C.M.; Vis, D.J.; Jellema, R.H.; Pijl, H.; Roelfsema, F.; van der Greef, J. Dynamic metabolomic data analysis: A tutorial review. Metabolomics 2010, 6, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Jia, D.; Lin, Z.; Guo, B.; He, B.; Lu, C.; Xiao, C.; Liu, Z.; Zhao, N.; Bian, Z.; et al. Potential metabolic biomarkers to identify interstitial lung abnormalities. Int. J. Mol. Sci. 2016, 17, 1148–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.C.; Sun, C.H.; Liu, L.Y.; Sun, X.H.; Jin, X.W.; Song, W.L.; Liu, X.Q.; Wan, X.L. Serum fatty acid profiles using GC-MS and multivariate statistical analysis: Potential biomarkers of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Blekherman, G.; Laubenbacher, R.; Cortes, D.F.; Mendes, P.; Torti, F.M.; Akman, S.; Torti, S.V.; Shulaev, V. Bioinformatics tools for cancer metabolomics. Metabolomics 2011, 7, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chen, T.; Ding, S.; Yang, G.; Xu, Z.; Xu, K.; Zhang, S.; Ma, T.; Zhang, J. Metabolomic study of the bone trabecula of osteonecrosis femoral head patients based on UPLC–MS/MS. Metabolomics 2016, 12, 48–62. [Google Scholar] [CrossRef]
- Wu, C.; Lei, R.; Tiainen, M.; Wu, S.; Zhang, Q.; Pei, F.; Guo, X. Disordered glycometabolism involved in pathogenesis of Kashin-Beck disease, an endemic osteoarthritis in China. Exp. Cell Res. 2014, 326, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, S.; Qiu, Y.; Zhao, T.; Chen, T.; Su, M.; Chu, L.; Lv, A.; Liu, P.; Jia, W. Urinary metabolomics as a potentially novel diagnostic and stratification tool for knee osteoarthritis. Metabolomics 2009, 6, 109–118. [Google Scholar] [CrossRef]
- Zhai, G.; Wang-Sattler, R.; Hart, D.J.; Arden, N.K.; Hakim, A.J.; Illig, T.; Spector, T.D. Serum branched-chain amino acid to histidine ratio: A novel metabolomic biomarker of knee osteoarthritis. Ann. Rheum. Dis. 2010, 69, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, A.; Kleerekoper, M.; Bayraktar, M.; Kucuk, O. Soy isoflavones in the management of postmenopausal osteoporosis. Menopause 2008, 15, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: A lifecourse approach. J. Bone Miner. Res. 2014, 29, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Al-Anazi, A.F.; Qureshi, V.F.; Javaid, K.; Qureshi, S. Preventive effects of phytoestrogens against postmenopausal osteoporosis as compared to the available therapeutic choices: An overview. J. Nat. Sci. Biol. Med. 2011, 2, 154–163. [Google Scholar] [PubMed]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.A.; Overgaard, K.; Riis, B.J.; Christiansen, C. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. Br. Med. J. 1991, 303, 961–964. [Google Scholar] [CrossRef]
- Seibel, M.J. Biochemical markers of bone metabolism in the assessment of osteoporosis: Useful or not? J. Endocrinol. Investig. 2003, 26, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Hlaing, T.T.; Compston, J.E. Biochemical markers of bone turnover-uses and limitations. Ann. Clin. Biochem. 2014, 51, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.Z.; Wu, X.P.; Liu, S.P.; Luo, X.H.; Cao, X.Z.; Xie, H.; Liao, E.Y. Age-related changes in bone biochemical markers and their relationship with bone mineral density in normal Chinese women. J. Bone Miner. Metab. 2006, 24, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Patrick, G.; Dennis, M.; Francoise, M.; Elisabeth, S.R.; Pierre, D.D. Long-Term variability of markers of bone turnover in postmenopausal women and implications for their clinical use: The OFELY study. J. Bone Miner. Res. 2003, 18, 1789–1794. [Google Scholar]
- Ivaska, K.K.; Lenora, J.; Gerdhem, P.; Akesson, K.; Väänänen, H.K.; Obrant, K.J. Serial assessment of serum bone metabolism markers identifies women with the highest rate of bone loss and osteoporosis risk. J. Clin. Endocr. Metab. 2008, 93, 2622–2632. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D.J.; Alexandersen, P.; Karsdal, M.A.; Qvist, P.; Schaller, S.; Tanko, L.B. An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur. J. Clin. Pharmacol. 2006, 62, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D.; Eastell, R.; Garnero, P.; Seibel, M.J.; Stepan, J. The use of biochemical markers of bone turnover in osteoporosis. Osteoporos. Int. 2000, 11, S2–S17. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Bao, J.; An, G.; Ouyang, G.; Zhang, P.; Wang, C.; Ying, H.; Ouyang, P.; Ma, B.; Zhang, Q. Association between the metabolome and bone mineral density in pre- and post-menopausal Chinese women using GC–MS. Mol. BioSyst. 2016, 12, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, X.; Zhao, L.; Li, F.; Xiong, Z. Kidney tissue targeted metabolic profiling of glucocorticoid-induced osteoporosis and the proposed therapeutic effects of Rhizoma Drynariae studied using UHPLC/MS/MS. Biomed. Chromatogr. 2014, 28, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Rotatori, S.; Bianciardi, S.; Gonnelli, S.; Nuti, R.; Merlotti, D. Appropriate models for novel osteoporosis drug discovery and future perspectives. Expert Opin. Drug Dis. 2015, 10, 1201–1216. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, X.; Chen, Z.; Ma, X.; Li, D.; Shang, P.; Qian, A. Neuropeptide FF attenuates RANKL-induced differentiation of macrophage-like cells into osteoclast-like cells. Arch. Oral Biol. 2015, 60, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Cheng, M.; Zhang, X.; Xiao, H. A metabolomics study of the inhibitory effect of 17-β-estradiol on osteoclast proliferation and differentiation. Mol. BioSyst. 2015, 11, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Kalu, D.N. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991, 15, 175–192. [Google Scholar] [CrossRef]
- Canalis, E.; Mazziotti, G.; Giustina, A.; Bilezikian, J.P. Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteoporos. Int. 2007, 18, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.P.; Gerszten, R.E. Metabolomics and cardiovascular biomarker discovery. Clin. Chem. 2012, 58, 139–147. [Google Scholar] [CrossRef] [PubMed]
- You, Y.S.; Lin, C.Y.; Liang, H.J.; Lee, S.H.; Tsai, K.S.; Chiou, J.M.; Chen, Y.C.; Tsao, C.K.; Chen, J.H. Association between the metabolome and low bone mineral density in Taiwanese women determined by 1H NMR spectroscopy. J. Bone Miner. Res. 2014, 29, 212–222. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.C.; Moco, S.; Lommen, A.; Keurentjes, J.J.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, D. Current trends and challenges in sample preparation for global metabolomics using liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 1523–1548. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Lison, D.; Junot, C.; Heilier, J.F. Applications of liquid chromatography coupled to mass spectrometry-based metabolomics in clinical chemistry and toxicology: A review. Clin. Biochem. 2011, 44, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liu, J.; Zhang, Q.; Ying, H.; A, J.; Sun, J.; Wu, D.; Wang, Y.; Li, J.; Liu, Y. Metabolomic profiles delineate signature metabolic shifts during estrogen deficiency-induced bone loss in rat by GC-TOF/MS. PLoS ONE 2013, 8, e54965. [Google Scholar] [CrossRef] [PubMed]
- Long, W.F.; Li, L.; Chen, H.Q.; Tang, Y.; He, X.L.; Zhou, J.R. 1H NMR-based metabonomics analysis of plasma from osteoporotic rats induced by ovariectomy. Med. Sci. Ed. 2009, 40, 843–847. [Google Scholar]
- Liu, Y.R.; Huang, R.Q.; Xiao, B.K.; Yang, J.Y.; Dong, J.X. 1H NMR metabolic profiling analysis offers evaluation of Nilestriol treatment in ovariectomised rats. Mol. Cell. Endocrinol. 2014, 387, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Yu, H.T.; Kao, J.P.; Yang, C.C.; Chiang, S.S.; Mishchuk, D.O.; Mau, J.L.; Slupsky, C.M. An NMR metabolomic study on the effect of alendronate in ovariectomized mice. PLoS ONE 2014, 9, e106559. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.R.; Xiao, B.K.; Yang, J.Y.; Guo, C.H.; Shen, S.J.; Tang, Z.S.; Dong, J.X.; Huang, R.Q. 1H NMR and HPLC–MS/MS-based global/targeted metabolomic evaluation of Hypericum perforatum L. intervention for menopause. J. Funct. Foods 2015, 17, 722–741. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kim, H.Y.; Singh, D.; Yeo, S.H.; Baek, S.Y.; Park, Y.K.; Lee, C.H. Metabolite profiling reveals the effect of dietary Rubus coreanus vinegar on ovariectomy-induced osteoporosis in a rat model. Molecules 2016, 21, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.; Lu, X.; Zheng, S.; Li, F.; Xiong, Z. Metabonomic study on the anti-osteoporosis effect of Rhizoma Drynariae and its action mechanism using ultra-performance liquid chromatography-tandem mass spectrometry. J. Ethnopharmacol. 2012, 139, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bo, Y.; Wu, X.; Wang, Q.; Qin, F.; Zhao, L.; Xiong, Z. An intergated serum and urinary metabonomic research based on UPLC-MS and therapeutic effects of Gushudan on prednisolone-induced osteoporosis rats. J. Chromatogr. B 2016, 1027, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol. 2006, 24, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Aboud, O.A.; Weiss, R.H. New opportunities from the cancer metabolome. Clin. Chem. 2013, 59, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Li, X.; Zhang, Q.; Wu, D.; Wang, G.; A, J.; Sun, J.; Li, J.; Liu, Y.; Wang, Y.; et al. Metabonomic profiling in studying anti-osteoporosis effects of strontium fructose 1,6-diphosphate on estrogen deficiency-induced osteoporosis in rats by GC/TOF-MS. Eur. J. Pharmacol. 2013, 718, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Liu, X.; Jiao, L.; Xu, C.; Zhang, Z.; Wang, L.; Li, Y.; Yang, C.; Zhang, W.; Sun, Y. Metabolomic evaluation of the response to endocrine therapy in patients with prostate cancer. Eur. J. Pharmacol. 2014, 729, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, B.H. The role of phytoestrogens in the prevention and treatment of osteoporosis in ovarian hormone deficiency. J. Am. Coll. Nutr. 2001, 20, 398S–402S. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, X.; He, P.; Cao, B.; Lv, Y.; Zhang, W.; Ni, X. Metabolomics in serum of ovariectomised rats and those exposed to 17 β-oestradiol and genistein. Gynecol. Endocrinol. 2010, 26, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Kusamori, K.; Katsumi, H.; Abe, M.; Ueda, A.; Sakai, R.; Hayashi, R.; Hirai, Y.; Quan, Y.S.; Kamiyama, F.; Sakane, T.; et al. Development of a novel transdermal patch of alendronate, a nitrogen-containing bisphosphonate, for the treatment of osteoporosis. J. Bone Miner. Res. 2010, 25, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, Q.; Wu, D.; Wang, Y.L.; Hu, Y.Y.; Cheng, Y.P.; Yang, Z.D.; Zheng, Y.Y.; Ying, H.J. Strontium fructose 1,6-diphosphate prevents bone loss in a rat model of postmenopausal osteoporosis via the OPG/RANKL/RANK pathway. Acta Pharmacol. Sin. 2012, 33, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wang, Y.; Liu, L.; Zhao, L.; Han, T.; Zhang, Q.; Qin, L. A 1HNMR-based metabonomics study of postmenopausal osteoporosis and intervention effects of Er-Xian Decoction in ovariectomized rats. Int. J. Mol. Sci. 2011, 12, 7635–7651. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, Q.; Wang, G.; A, J.; Wu, D.; Liu, Y.; Cao, B.; Liu, L.; Hu, Y.; Wang, Y.; et al. GC-TOF/MS-based metabolomic profiling of estrogen deficiency-induced obesisty in ovariectomized rats. Acta Pharmacol. Sin. 2011, 32, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Jiang, Y.; Han, T.; Zhang, N.; Qin, L.; Xin, H.; Zhang, Q. Comparative proteomic and metabolomic analysis reveal the antiosteoporotic molecular mechanism of icariin from Epimedium brevicornu Maxim. J. Ethnopharmacol. 2016, 192, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, L.; Lin, Z.; Li, J.; Li, H.; Sun, J.; Zhu, K.; Yu, Z.; Xu, K.; Yang, Q.; et al. Metabonomics study of the anti-osteoporosis effect of velvet collagen hydrolysate using rapid resolution liquid chromatography combined with quadrupole time-of-flight tandem mass spectrometry. J. Liq. Chromatogr. Relat. Technol. 2014, 38, 117–122. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Zheng, S.; Jiang, M.; Xin, C.; Lu, X.; Li, F.; Xiong, Z. Metabonomic study on protective effect of ethanol extracts of Drynariae rhizoma on osteoporosis in rats urine by using UPLC–MS/MS. China J. Chin. Mater. Medica 2012, 37, 658–662. [Google Scholar]
- Cao, H.; Zhang, A.; Zhang, H.; Sun, H.; Wang, X. The application of metabolomics in traditional Chinese medicine opens up a dialogue between Chinese and Western medicine. Phytother. Res. 2015, 29, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lamers, R.J.; Korthout, H.A.; van Nesselrooij, J.H.; Witkamp, R.F.; van der Heijden, R.; Voshol, P.J.; Havekes, L.M.; Verpoorte, R.; van der Greef, J. Metabolomics in the context of systems biology: Bridging traditional Chinese medicine and molecular pharmacology. Phytother. Res. 2005, 19, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, Z.; Sun, W.; Wang, P.; Wang, X. Metabolomics: Towards understanding traditional Chinese medicine. Planta Med. 2010, 76, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Lee, K.S.; Luk, H.K.; Wan, H.Y.; Ho, C.K.; Zhang, Y.; Wong, M.S. Er-xian Decoction exerts estrogen-like osteoprotective effects in vivo and in vitro. Am. J. Chin. Med. 2014, 42, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wang, Y.; Jiang, Y.; Han, T.; Nie, Y.; Zhao, L.; Zhang, Q.; Qin, L. Comparative effects of er-xian decoction, epimedium herbs, and icariin with estrogen on bone and reproductive tissue in ovariectomized rats. Evid.-Based Complement. Altern. 2012, 2012, 241416. [Google Scholar]
- Qin, L.; Han, T.; Zhang, Q.; Cao, D.; Nian, H.; Rahman, K.; Zheng, H. Antiosteoporotic chemical constituents from Er-Xian Decoction, a traditional Chinese herbal formula. J. Ethnopharmacol. 2008, 118, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Nian, H.; Qin, L.P.; Zhang, Q.Y.; Zheng, H.C.; Yu, Y.; Huang, B.K. Antiosteoporotic activity of Er-Xian Decoction, a traditional Chinese herbal formula, in ovariectomized rats. J. Ethnopharmacol. 2006, 108, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, H.; Lu, X.; Li, C.; Xiong, Z.; Li, F. Simultaneous determination of icariin, icariside II and osthole in rat plasma after oral administration of the extract of Gushudan (a Chinese compound formulation) by LC-MS/MS. J. Chromatogr. B 2007, 860, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.C.; Lee, J.W.; Yoon, C.H.; Lee, Y.C.; Chung, K.H.; Kim, M.G.; Kim, C.H. Stimulative effects of Drynariae Rhizoma extracts on the proliferation and differentiation of osteoblastic MC3T3-E1 cells. J. Ethnopharmacol. 2005, 96, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, K.S.; Choi, B.J.; Chung, K.H.; Chang, Y.C.; Lee, S.D.; Park, K.K.; Kim, H.M.; Kim, C.H. Anti-bone resorption activity of deer antler aqua-acupunture, the pilose antler of Cervus korean TEMMINCK var. mantchuricus Swinhoe (Nokyong) in adjuvant-induced arthritic rats. J. Ethnopharmacol. 2005, 96, 497–506. [Google Scholar] [PubMed]
- Li, Y.J.; Kim, T.H.; Kwak, H.B.; Lee, Z.H.; Lee, S.Y.; Jhon, G.J. Chloroform extract of deer antler inhibits osteoclast differentiation and bone resorption. J. Ethnopharmacol. 2007, 113, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Mikiya, K.; Masahiro, F.; Yoshinori, T.; Takashi, N.; Kazushige, T.; Naoyuki, N. Enhancing effect of dietary vinegar on the intestinal absorbtion of calcium in ovariectomized rats. Biosci. Biotechnol. Biochem. 1999, 63, 905–910. [Google Scholar]
- Dawson-Hughes, B.; Harris, S.S.; Krall, E.A.; Dallal, G.E. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N. Engl. J. Med. 1997, 337, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 1992, 327, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, J.; Takeda, T.; Shoichi, I. Effect of exercise training and detraining on bone mineral density in postmenopausal women with osteoporosis. J. Orthop. Sci. 2001, 6, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, J.R.; Gooley, P.R.; Nahid, A.; Tull, D.L.; McConville, M.J.; Kukuljan, S.; Nowson, C.A.; Daly, R.M.; Ebeling, P.R. 1H NMR analysis of the human urinary metabolome in response to an 18-month multi-component exercise program and calcium-vitamin-D3 supplementation in older men. Appl. Physiol. Nutr. Metab. 2014, 39, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Kukuljan, S.; Nowson, C.A.; Sanders, K.M.; Nicholson, G.C.; Seibel, M.J.; Salmon, J.; Daly, R.M. Independent and combined effects of calcium-vitamin D3 and exercise on bone structure and strength in older men: An 18-month factorial design randomized controlled trial. J. Clin. Endocrinol. Metab. 2011, 96, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Kukuljan, S.; Nowson, C.A.; Bass, S.L.; Sanders, K.; Nicholson, G.C.; Seibel, M.J.; Salmon, J.; Daly, R.M. Effects of a multi-component exercise program and calcium-vitamin-D3-fortified milk on bone mineral density in older men: A randomised controlled trial. Osteoporos. Int. 2009, 20, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Current progress in computational metabolomics. Brief Bioinform. 2007, 8, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Bryzgalova, G.; Lundholm, L.; Portwood, N.; Gustafsson, J.Å.; Khan, A.; Efendic, S.; Dahlman-Wright, K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E904–E912. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Chen, C.H.; Cheng, Y.C.; Chang, C.H.; Lee, C.T.; Chang, J.K.; Cheng, J.T.; Chang, F.M. Glucosamine-induced insulin resistance in ovariectomized rats is relevant to decreasing the expression of glucose transport protein subtype 4 in the skeletal muscle and in increasing the size of pancreatic islets. Menopause 2012, 19, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Pirimoglu, Z.M.; Arslan, C.; Buyukbayrak, E.E.; Kars, B.; Karsidag, Y.K.; Unal, O.; Turan, M.C. Glucose tolerance of premenopausal women after menopause due to surgical removal of ovaries. Climacteric 2011, 14, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Motyl, K.J.; McCabe, L.R.; Schwartz, A.V. Bone and glucose metabolism: A two-way street. Arch. Biochem. Biophys. 2010, 503, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.B. Bone: From a reservoir of minerals to a regulator of energy metabolism. Kidney Int. 2011, 79, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, M.E.; Gimble, J.M. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr. Opin. Pharmacol. 2004, 4, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Sugimota, T.; Yano, S.; Yamauchi, M.; Sowa, H.; Chen, Q.; Chihara, K. Plasma lipids and osteoporosis in postmenopausal women. Endocr. J. 2002, 49, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.H.; Lee, M.Y.; Kim, S.J.; Joe, S.G.; Kim, G.B.; Kim, I.S.; Kim, N.S.; Hong, C.U.; Kim, S.Z.; Kim, J.S.; et al. Taurine increases cell proliferation and generates an increase in [Mg2+]i accompanied by ERK 1/2 activation in human osteoblast cells. FEBS Lett. 2007, 581, 5929–5934. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Sjogren, K. Effects of the gut microbiota on bone mass. Trends Endocrinol. Metab. 2015, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Steves, C.J.; Bird, S.; Williams, F.M.; Spector, T.D. The microbiome and musculoskeletal conditions of aging: A review of evidence for impact and potential therapeutics. J. Bone Miner. Res. 2016, 31, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, K.; Engdahl, C.; Henning, P.; Lerner, U.H.; Tremaroli, V.; Lagerquist, M.K.; Backhed, F.; Ohlsson, C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012, 27, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.A.; Ljunggren, O.; Stehman-Breen, C.; Cummings, S.R.; McClung, M.R.; Goemaere, S.; Ebeling, P.R.; Franek, E.; Yang, Y.C.; Egbuna, O.I.; et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J. Bone Miner. Res. 2011, 26, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xiong, Z.; Li, J.; Zheng, S.; Huo, T.; Li, F. Metabonomic study on 'Kidney-Yang Deficiency syndrome' and intervention effects of Rhizoma Drynariae extracts in rats using ultra performance liquid chromatography coupled with mass spectrometry. Talanta 2011, 83, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Traynor, J.; Mactier, R.; Geddes, C.C.; Fox, J.G. How to measure renal function in clinical practice. Br. Med. J. 2006, 7571, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Gagné, E.R.; Deland, E.; Daudon, M.; Noël, L.H.; Nawar, T. Chronic renal failure secondary to 2,8-dihydroxyadenine deposition: The first report of recurrence in a kidney transplant. Am. J. Kidney Dis. 1994, 24, 104–107. [Google Scholar] [CrossRef]
- Salmén, T.; Heikkinen, A.M.; Mahonen, A.; Kröger, H. Early postmenopausal bone loss is associated with PvuII estrogen receptor gene polymorphism in Finnish women: Effect of hormone replacement therapy. J. Bone Miner. Res. 2000, 15, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Koh, L.K.H.; Sedrine, W.B.; Torralba, T.P.; Kung, A.; Fujiwara, S.; Chan, S.P.; Huang, Q.R.; Rajatanavin, R.; Tsai, K.S.; Park, H.M.; et al. A simple tool to identify Asian women at increased risk of osteoporosis. Osteoporos. Int. 2001, 12, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.L.; Slemenda, C.W.; Johnston, C.C., Jr. The contribution of bone loss to postmenopausal osteoporosis. Osteoporos. Int. 1990, 1, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Mishur, R.J.; Rea, S.L. Applications of mass spectrometry to metabolomics and metabonomics: Detection of biomarkers of aging and of age-related diseases. Mass Spectrom. Rev. 2012, 31, 70–95. [Google Scholar] [CrossRef] [PubMed]
- Madsen, R.; Lundstedt, T.; Trygg, J. Chemometrics in metabolomics-a review in human disease diagnosis. Anal. Chim. Acta 2010, 659, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Sambrook, P.N.; Eisman, J.A. Bone loss, physical activity, and weight change in elderly women: The Dubbo osteoporosis epidemiology study. J. Bone Miner. Res. 1998, 13, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Espinoza, J.; Gotsch, F.; Kusanovic, J.P.; Friel, L.A.; Erez, O.; Mazaki-Tovi, S.; Than, N.G.; Hassan, S.; Tromp, G. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG-Int. J. Obstet. Gynaecol. 2006, 113, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Blanchet, L.; Buydens, L.M.; Wijmenga, S.S. NMR and pattern recognition methods in metabolomics: From data acquisition to biomarker discovery: A review. Anal. Chim. Acta 2012, 750, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Shum, L.C.; White, N.S.; Nadtochiy, S.M.; Bentley, K.L.; Brookes, P.S.; Jonason, J.H.; Eliseev, R.A. Cyclophilin D knock-out mice show enhanced resistance to osteoporosis and to metabolic changes observed in aging bone. PLoS ONE 2016, 11, e0155709. [Google Scholar] [CrossRef] [PubMed]
- Elnenaei, M.O.; Chandra, R.; Mangion, T.; Moniz, C. Genomic and metabolomic patterns segregate with responses to calcium and vitamin D supplementation. Br. J. Nutr. 2011, 105, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Horgan, R.P.; Kenny, L.C. ‘Omic’ technologies: Genomics, transcriptomics, proteomics and metabolomics. Obstet. Gynaecol. 2011, 13, 189–195. [Google Scholar] [CrossRef]
- Ghosh, N.; Dutta, M.; Singh, B.; Banerjee, R.; Bhattacharyya, P.; Chaudhury, K. Transcriptomics, proteomics and metabolomics driven biomarker discovery in COPD: An update. Expert Rev. Mol. Diagn. 2016, 16, 897–913. [Google Scholar] [CrossRef] [PubMed]
| Detection Method | Biological Sample | Sample Source | Sample Preparation Procedures | References |
|---|---|---|---|---|
| GC/MS | Plasma | OVX SD rats | Deproteinization: plasma:methanol = 1:4 (v/v), containing the internal standard myristic-1, 2-13C2 acid | [34] |
| Centrifugation: 12,000× g for 10 min | ||||
| Dryness: under vacuum in speedvac concentrator | ||||
| Methoxylation: added methoxyamine in pyridine and performed at room temperature for 16 h | ||||
| Derivation: added MSTFA with 1% TMCS and reacted at room temperature for 1 h | ||||
| Addition of external standard: added heptane containing methyl myristate | ||||
| Serum | Human | Deproteinization: serum:methanol = 1:4 (v/v), containing the internal standard myristic-1, 2-13C2 acid | [22] | |
| Centrifugation: 20,000× g for 10 min | ||||
| Dryness: under vacuum in speedvac concentrator | ||||
| Methoxylation: added methoxyamine in pyridine and incubated at room temperature for 16 h | ||||
| Derivation: added MSTFA with 1% TMCS and reacted at room temperature for 1 h | ||||
| Addition of external standard: added heptane containing methyl myristate | ||||
| 1H NMR | Plasma | OVX SD rats | Mixture: TSP:plasma:D2O = 1:3:2 (v/v/v) | [35] |
| Centrifugation: 14,000 rpm for 8 min | ||||
| Serum | OVX SD rats | Centrifugation: 14,000 rpm for 8 min | [36] | |
| Addition of internal standard: final DSS concentration to1.0 mM | ||||
| Serum | OVX C57BL/6JNarl mice | Centrifugation: at maximum speed for 20 min | [37] | |
| Filtration: filtered through Amicon 3000 molecular weight cutoff filters | ||||
| Addition of internal standard: final DSS concentration to 4.8 mM | ||||
| pH value adjustment: 6.8 | ||||
| Urine | OVX SD rats | Dilution: urine:PBS = 1:1 (v/v) | [36,38] | |
| Centrifugation: 14,000 rpm for 8 min | ||||
| Addition of internal standard: final DSS concentration to 0.5 mM | ||||
| LC/MS | Plasma | Prednisolone induced Wistar rats/OVX SD rats | Deproteinization: plasma:acetonitrile/methanol = 1:2/1:3 (v/v), mixed and vortexed | [39,40] |
| Homogenization: homogenized for 3 min by using a mixer mill and was kept at −20 °C for 1 h | ||||
| Centrifugation: 13,000/12,000 rpm for 10 min, passed through a 0.2 µm PTFE filter | ||||
| Dryness: at 4 °C under a gentle stream of nitrogen/dried with a speed vacuum machine | ||||
| Reconstitution: reconstituted in acetonitrile-water (1:9, v/v), vortexed for 30 s/dissolved in methanol | ||||
| Filtration: syringe-filtered | ||||
| Serum | Prednisolone induced SD rats | Deproteinization: plasma:acetonitrile = 1:3 (v/v), mixed and vortexed | [41] | |
| Centrifugation: 13,000 rpm for 10 min | ||||
| Dryness: under a gentle stream of nitrogen | ||||
| Reconstitution: reconstituted in acetonitrile-water (1:9, v/v), vortexed for 30 s | ||||
| Urine | Prednisolone induced SD rats | Dilution: urine: water = 1:1 (v/v) | [41] | |
| Centrifugation: at 13,000 rpm for 10 min | ||||
| Filtration: filtered through 0.22 um membrane filter | ||||
| Cell extracts | RNAKL induced Mouse RAW 264.7 | Cell quenching: washed with PBS, scraped, centrifuged, washed, resuspended in water | [26] | |
| Cell disruption: using an Ultrasonic cell pulverizer | ||||
| Centrifugation: at 12,000 rpm for 10 min | ||||
| Extraction: using cold methanol/water (4:1, v/v) to extract metabolites | ||||
| Centrifugation: at 12,000 rpm for 10 min | ||||
| Dryness: under vacuum | ||||
| Reconstitution: resuspended in 5% acetonitrile | ||||
| Kidney tissue extracts | Prednisolone induced Wistar rats | Homogenization: tissue:methanol = 125:1(mg/mL) in ice-water bath | [23] | |
| Centrifugation: 13,000 rpm for 10 min at 4 °C | ||||
| Extraction: supernatant:water = 1:2 (v/v), vortexed, chloroform: methanol = 2:1 (v/v) added | ||||
| Centrifugation: 3500 rpm for 10 min | ||||
| Dryness: 40 °C under nitrogen | ||||
| Reconstitution: dissolved in methanol, vortexed, centrifuged at 13,000 rpm for 10 min |
| Biological Sample | Sample Source | Detective Method | Change Trend in Osteoporosis Group | Related Metabolic Pathways | References |
|---|---|---|---|---|---|
| Plasma | Prednisolone induced Wistar rats | LC/MS | ↑ LysoPCs (C16:0, C18:0, C18:1 and C18:2), phenylalanine, tryptophane | Oxidative system, tryptophane metabolism, phenylalanine metabolism | [40] |
| OVX SD rats | GC/MS | ↑ arachidonic acid, actadecadienoic acid, valine, leucine, isoleucine, homocysteine, hydroxyproline, 3-hydroxybutyric acid ↓ docosahexaenoic acid, dodecanoic acid, lysine | Fatty acid metabolism, amino acid metabolism | [34] | |
| OVX SD rats | 1H NMR | ↑ lactate, acetone, ethonal ↓ glucose, choline/phosphatidylcholine, vLDL/LDL, HDL/LDL, alanine, lipoprotein, fatty acid | Glucose metabolism, lipid metabolism | [35] | |
| OVX SD rats | 1H NMR | ↑ LDL/vLDL, choline, lactate, lipids, acetoacetate ↓ alanine | Lipid metabolism, amino acid metabolism, energy metabolism, oxidative system | [51] | |
| Postmenopausal woman | 1H NMR | ↑ acetate, glutamine ↓ glucose, vLDLs, lactate, acetone, lipids, | Pyruvate metabolism, fatty acid metabolism, carbohydrate metabolism, d-glutamine and d-glutamate metabolism | [30] | |
| Serum | OVX C57BL/6JNarl mice | 1H NMR | ↑ 2-oxoglutarate, fumarate, taurine, glucose ↓ dimethylamine, allantoin, ethanol, glycine, citrate, succinate, malate, 3-hydroxybutyrate, acetate | Energy metabolism, TCA cycles, amino acid metabolism | [37] |
| OVX SD rats | LC/MS | ↑ arachidonic acid ↓ eicosapentaenoic acid, ergocalciferol, cholecalciferol | Lipid and fatty acid metabolism | [48] | |
| OVX SD rats (6 weeks post-surgery) | GC/MS | ↑ cholesterol, glycerol, octadecadienoic acid, 3-hydroxy-butanoic acid, glucose, isoleucine, valine, leucine, glycie ↓ glyceraldehyde 3-phosphate, alanine, arabinofuranose | Glucose metabolism, lipid metabolism, amino acid metabolism | [52] | |
| Prednisolone induced SD rats | LC/MS | ↑ arginine, valine, phenylalanine, tryptophan lypsoPCs (C20:4, C16:0, C18:1, and C18:0) ↓ creatine | Amino acid and lipid metabolism | [41] | |
| OVX SD rats | 1H NMR | ↑ acetate, betaine, carnitine, choline, creatine, creatinine, glycine, glucose, glutamate, histidine, lysine, ornithine, proline ↓ 3-hydroxybutyrate, alanine, formate, glutamine, taurine, threonine | Glycolysis and gluconeogenesis, methionine cycle, fatty acid metabolism, one-carbon unit pathways, urea cycle | [36] | |
| OVX ICR mice | 1H NMR | ↑ LDL/vLDL, glucose, lactate, lipids, NAc/OAc | Lipid and energy metabolism | [53] | |
| Postmenopausal with osteoporosis | GC/MS | ↑ linoleic acid, oleic acid, arachidonic acid, 11, 14-eicosadienoic acid, eicosapentaenoic acid, tryptophan ↓ 3-hydroxy-l-proline | Lipid metabolism, amino acid metabolism, energy metabolism | [22] | |
| Urine | Dexamethasone induced SD rats | LC/MS | ↑ tryptophan, asparagines, arginine, GPCho ↓ taurine, saccharopine, glucose, leucine | Amino acid and phospholipid metabolism | [54] |
| Prednisolone induced Wistar rats | LC/MS | ↑ phenylalanine, creaol sulfate, phenaceturic acid ↓ creatinine, citric acid, azelaic acid, hippurate, tryptophan, indoxyl sulfate | Amino acid metabolism, energy metabolism, gut microflora, oxidative system | [55] | |
| OVX C57BL/6JNarl mice | 1H NMR | ↑ glucose, acetyl-glucoprotein, glycine ↓ isoleucine, glutamate, glucose | Carbohydrate metabolism, lipid metabolism, amino acid metabolism | [51] | |
| OVX SD rats | 1H NMR | ↑ 3-indoxylsulfate, allantoin, betaine, carnitine, creatinine, glutamine, glycine, hippurate, lysine, methylhistidine, β-alanine ↓ 2-oxoglutarate, acetate, citrate, fumarate, methionine, N,N-dimethylglycine, succinate, taurine, TMAO | TCA cycle, methionine cycle, fatty acid metabolism, one-carbon unit pathways, urea cycle | [36] | |
| Kidney tissue | Prednisolone induced Wistar rats | LC/MS | ↑ phenylalanine, lypsoLCs (C16:0 and C18:0), dihydrosphingosines (C16 and C18), phytosphingosines (C18 and C20) | Phenylalanine metabolism, sphingolipid metabolism, amino acid metabolism, kidney damage | [23] |
| Classifications | Treatments | Sample Sources | Biological Samples | Purposes | References |
|---|---|---|---|---|---|
| Estrogen and estrogen derivatives | Nilestriol | OVX SD rats | Serum/urine | Regulation of estrogen deficiency disorder | [36] |
| 17-β-Estradiol | Mouse osteoclast cells | Cell extracts | Inhibition of the activity of osteoclasts | [26] | |
| 17-β-Estradiol | OVX SD rats | Serum | Improvement of estrogen deficiency status | [48] | |
| Genistein | OVX SD rats | Serum | Improvement of estrogen deficiency status | [48] | |
| Bisphosphonates | FDP-Sr | OVX SD rats | Plasma | Anti-osteoporosis efficacy | [45] |
| Fosamax | OVX C57BL/6JNarl mice | Serum | Anti-osteoporosis efficacy | [37] | |
| TCM or herbal formula extracts | Rhizoma Drynariae extract | Prednisolone induced Wistar rats | Plasma | Anti-osteoporosis efficacy | [40] |
| Rhizoma Drynariae extract | Prednisolone induced Wistar rats | Kidney tissue extracts | Anti-osteoporosis and replenish the kidney | [23] | |
| Rhizoma Drynariae extract | Prednisolone induced rats | Urine | Anti-osteoporosis efficacy | [55] | |
| Gushudan extract | Prednisolone induced SD rats | Serum/urine | Anti-osteoporosis efficacy | [41] | |
| Er-Xian decoction | OVX SD rats | Plasma/urine | Anti-osteoporosis efficacy | [51] | |
| Hypericum perforatum L. extract | OVX SD rats | Serum/urine | Relieve menopausal syndromes | [38] | |
| Velvet collagen hydrolysate | Dexamethasone induced SD rats | Urine | Anti-osteoporosis efficacy | [54] | |
| Rubus coteanus Vinegar | OVX SD rats | Plasma | Anti-osteoporosis efficacy | [39] | |
| Icariin from Epimedii Folium | OVX ICR mice | Serum | Anti-osteoporosis efficacy | [53] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, H.; Jiang, F.; Guan, D.; Lu, C.; Guo, B.; Chan, C.; Peng, S.; Liu, B.; Guo, W.; Zhu, H.; et al. Metabolomics and Its Application in the Development of Discovering Biomarkers for Osteoporosis Research. Int. J. Mol. Sci. 2016, 17, 2018. https://doi.org/10.3390/ijms17122018
Lv H, Jiang F, Guan D, Lu C, Guo B, Chan C, Peng S, Liu B, Guo W, Zhu H, et al. Metabolomics and Its Application in the Development of Discovering Biomarkers for Osteoporosis Research. International Journal of Molecular Sciences. 2016; 17(12):2018. https://doi.org/10.3390/ijms17122018
Chicago/Turabian StyleLv, Huanhuan, Feng Jiang, Daogang Guan, Cheng Lu, Baosheng Guo, Chileung Chan, Songlin Peng, Baoqin Liu, Wenwei Guo, Hailong Zhu, and et al. 2016. "Metabolomics and Its Application in the Development of Discovering Biomarkers for Osteoporosis Research" International Journal of Molecular Sciences 17, no. 12: 2018. https://doi.org/10.3390/ijms17122018
APA StyleLv, H., Jiang, F., Guan, D., Lu, C., Guo, B., Chan, C., Peng, S., Liu, B., Guo, W., Zhu, H., Xu, X., Lu, A., & Zhang, G. (2016). Metabolomics and Its Application in the Development of Discovering Biomarkers for Osteoporosis Research. International Journal of Molecular Sciences, 17(12), 2018. https://doi.org/10.3390/ijms17122018