Comparative Analysis of Volatile Composition in Chinese Truffles via GC × GC/HR-TOF/MS and Electronic Nose
Abstract
:1. Introduction
2. Results
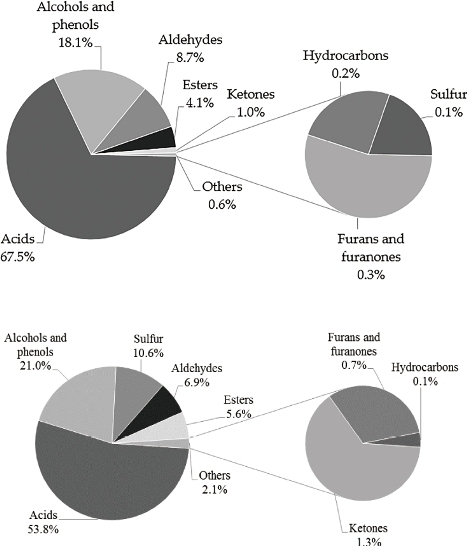
2.1. Comprehensive Two-Dimensional gas Chromatography (GC × GC)/High Resolution Time-of-Flight Mass Spectrometry (HR-TOF/MS) Analysis
2.2. Electronic Nose Response
3. Discussion
3.1. GC × GC/HR-TOF/MS Analysis
3.2. Electronic Nose Response
4. Materials and Methods
4.1. Chemicals and Samples
4.2. Extraction Methods
4.2.1. Direct Solvent Extraction
4.2.2. Solvent-Assisted Flavor Evaporation
4.3. GC × GC/HR-TOF/MS Analysis
4.4. Compounds Identification
4.5. Electronic Nose Analysis
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeandroz, S.; Murat, C.; Wang, Y.; Bonfante, P.; Tacon, F.L. Molecular phylogeny and historical biogeography of the genus Tuber, the ‘true truffles’. J. Biogeogr. 2008, 35, 815–829. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.G. Verification of Chinese names of truffles and their conservation in natural habitats. Plant Divers. Resour. 2011, 33, 625–642. [Google Scholar]
- Liu, B. New species and new records of hypogeous fungi from China (I). Acta Mycol. Sin. 1985, 4, 84–89. [Google Scholar]
- Luis, G.; Montero, G.; Díaz, P.; Massimo, G.D.; García-Abril, A. A review of research on Chinese Tuber species. Mycol. Prog. 2010, 9, 315–335. [Google Scholar]
- Yang, M.C. Truffles in Southwest China. In Proceedings du Ve Congrès International Science et Culture de la Truffe, Aix-en-Provence, Marseille, France, 4–6 March 1999.
- Splivallo, R.; Bossi, S.; Maffei, M.; Bonfante, P. Discrimination of truffle fruiting body versus mycelial aromas by stir bar sorptive extraction. Phytochemistry 2007, 68, 2584–2598. [Google Scholar] [CrossRef] [PubMed]
- Culleré, L.; Ferreira, V.; Venturini, M.E.; Marco, P.; Blanco, D. Potential aromatic compounds as markers to differentiate between Tuber melanosporum and Tuber indicum truffles. Food Chem. 2013, 141, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Bellesia, F.; Pinetti, A.; Bianchi, A.; Tirillini, B. Volatile compounds of the white truffle (Tuber magnatum Pico) from Middle Italy. Flavour Frag. J. 1996, 11, 239–243. [Google Scholar] [CrossRef]
- Bellesia, F.; Pinetti, A.; Tirillini, B.; Bianchi, A. Temperature-dependent evolution of volatile organic compounds in Tuber borchii from Italy. Flavour Frag. J. 2001, 16, 1–6. [Google Scholar] [CrossRef]
- Dı́az, P.; Ibanez, E.; Senorans, F.J.; Reglero, G.J. Truffle aroma characterization by headspace solid-phase microextraction. J. Chromatogr. A 2003, 1017, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Gioacchini, A.M.; Menotta, M.; Bertini, L.; Rossi, I.; Zeppa, S.; Zambonelli, A.; Piccoli, G.; Stocchi, V. Solid-phase microextraction gas chromatography/mass spectrometry: A new method for species identification of truffles. Rapid Commun. Mass Spectrom. 2005, 19, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Fanali, C.; Pennazza, G.; Tedone, L.; Dugo, L.; Santonico, M.; Sciarrone, D.; Cacciola, F.; Cucchiarini, L.; Dacha, M.; et al. Screening of volatile compounds composition of white truffle during storage by GC × GC-(FID/MS) and gas sensor array analyses. LWT-Food Sci. Technol. 2015, 60, 905–913. [Google Scholar] [CrossRef]
- Pacionia, G.; Cerretanib, L.; Procidac, G.; Cichelli, A. Composition of commercial truffle flavored oils with GC–MS analysis and discrimination with an electronic nose. Food Chem. 2014, 146, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Shimoda, M.; Wu, Y.; Osajima, Y. Aroma compounds from aqueous solution of haze (Rhus succedanea) honey determined by adsorptive column chromatography. J. Agric. Food Chem. 1996, 44, 3913–3918. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredient, 6th ed.; CRC press: New York, NY, USA, 2010. [Google Scholar]
- Ong, P.K.C.; Acree, T.E.; Lavin, E.H. Characterization of volatiles in rambutan fruit (Nephelium lappaceum L.). J. Agric. Food Chem. 1998, 46, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Jordán, M.J.; Margarı́a, C.A.; Shaw, P.E.; Goodner, K.L. Aroma active components in aqueous kiwi fruit essence and kiwi fruit puree by GC-MS and multidimensional GC/GC-O. J. Agric. Food Chem. 2002, 50, 5386–5390. [Google Scholar] [CrossRef] [PubMed]
- Varlet, V.; Knockaert, C.; Prost, C.; Serot, T. Comparison of odor-active volatile compounds of fresh and smoked salmon. J. Agric. Food Chem. 2006, 54, 3391–3401. [Google Scholar] [CrossRef] [PubMed]
- Menotta, M.; Gioacchini, A.M.; Amicucci, A.; Buffalini, M.; Sisti, D.; Stocchi, V. Headspace solid-phase microextraction with gas chromatography and mass spectrometry in the investigation of volatile organic compounds in an ectomycorrhizae synthesis system. Rapid Commun. Mass Spectrom. 2004, 18, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Splivallo, R.; Novero, M.; Bertea, C.M.; Bossi, S.; Bonfante, P. Truffle volatiles inhibit growth and induce an oxidative burst in Arabidopsis thaliana. New Phytol. 2007, 175, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Dı́az, P.; Señoráns, F.J.; Reglero, G.; Ibañez, E. Truffle aroma analysis by headspace solid phase microextraction. J. Agric. Food Chem. 2002, 50, 6468–6472. [Google Scholar] [CrossRef] [PubMed]
- Mauriello, G.; Marino, R.; D’Auria, M.; Cerone, G.; Rana, G.L. Determination of volatile organic compounds from truffles via SPME-GC-MS. J. Chromatogr. Sci. 2004, 42, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Pelusio, F.; Nilsson, T.; Montanarella, L.; Tilio, R.; Larsen, B.; Facchetti, S.; Madsen, J.O. Headspace solid-phase microextraction analysis of volatile organic sulfur compounds in black and white truffle aroma. J. Agric. Food Chem. 1995, 43, 2138–2143. [Google Scholar] [CrossRef]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation—A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Pang, X.L.; Guo, X.F.; Qin, Z.H.; Yao, Y.B.; Hu, X.S.; Wu, J.H. Identification of aroma-active compounds in Jiashi muskmelon juice by GC-O-MS and OAV calculation. J. Agric. Food Chem. 2012, 60, 4179–4185. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zheng, F.P.; Chen, H.T.; Liu, S.Y.; Gu, C.; Song, Z.Y.; Sun, B.G. Identification of volatile components in Chinese Sinkiang fermented camel milk using SAFE, SDE, and HS-SPME-GC/MS. Food Chem. 2011, 129, 1242–1252. [Google Scholar]
- Lapsongphon, N.; Yongsawatdigul, J.; Cadwallader, K.R. Identification and characterization of the aroma-impact components of Thai fish sauce. J. Agric. Food Chem. 2015, 63, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zheng, F.P.; Chen, H.T.; Huang, M.Q.; Xie, J.C.; Chen, F.; Sun, B.G. Analysis of volatiles in Dezhou Braised Chicken by comprehensive two-dimensional gas chromatography/high resolution-time of flight mass spectrometry. LWT-Food Sci. Technol. 2015, 60, 1235–1242. [Google Scholar] [CrossRef]
- Mo, X.L.; Xu, Y.; Fan, W.L. Characterization of aroma compounds in Chinese rice wine Qu by solvent-assisted flavor evaporation and headspace solid-phase microextraction. J. Agric. Food Chem. 2010, 58, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Marcone, M.F. The biochemistry and biological properties of the world’s most expensive underground edible mushroom: Truffles. Food Res. Int. 2011, 44, 2567–2581. [Google Scholar] [CrossRef]
- Splivallo, R.; Ebeler, S.E. Sulfur volatiles of microbial origin are key contributors to human-sensed truffle aroma. Appl. Microbiol. Biotechnol. 2015, 99, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- Splivallo, R.; Ottonello, S.; Mello, A.; Karlovsky, P. Truffle volatiles: From chemical ecology to aroma biosynthesis. New Phytol. 2011, 189, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Gioacchini, A.M.; Menotta, M.; Guescini, M.; Saltarelli, R.; Ceccaroli, P.; Amicucci, A.; Barbieri, E.; Giomaro, G.; Stocchi, V. Geographical traceability of Italian white truffle (Tuber lemagnatum Pico) by the analysis of volatile organic compounds. Rapid Commun. Mass Spectrom. 2008, 22, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ahn, H.J.; Yook, H.S.; Kim, K.S.; Rhee, M.S.; Ryu, G.H.; Byun, M.W. Color, flavor, and sensory characteristics of γ-irradiated salted and fermented anchovy sauce. Radiat. Phys. Chem. 2004, 69, 179–187. [Google Scholar]
- Teai, T.; Claude-Lafontaine, A.; Schippa, C.; Cozzolino, F. Volatile compounds in fresh pulp of pineapple (Ananas comosus [L.] Merr.) from French Polynesia. J. Essent. Oil Res. 2001, 13, 314–318. [Google Scholar] [CrossRef]
- Burgard, D.R.; Kuznicki, J. Chemometrics: Chemical and Sensory Data; CRC: Boca Raton, FL, USA, 1990. [Google Scholar]
- Rotsatchakul, P.; Chaiseri, S.; Cadwallader, K.R. Identification of characteristic aroma components of Thai fried chili paste. J. Agric. Food Chem. 2008, 56, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Rinaldi, M.; Locatelli, M.; Piana, G.; Travaglia, F.; Coïsson, J.D.; Marco Arlorio, M. Characterization of Muscat wines aroma evolution using comprehensive gas chromatography followed by a post-analytic approach to 2D contour plots comparison. Food Chem. 2013, 140, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ai, N.S.; Liu, H.L.; Wang, J.; Zhang, X.M.; Zhang, H.J.; Chen, H.T.; Huang, M.Q.; Liu, Y.G.; Zheng, F.P.; Sun, B.G. Triple-channel comparative analysis of volatile flavour composition in raw whole and skim milk via electronic nose, GC-MS and GC-O. Anal. Methods 2015, 7, 4278–4284. [Google Scholar] [CrossRef]






| a No. | b RI Exp | b RI Lit | Compound Name | c CAS No. | Library Match Factor | Black Truffle (BT) | White Truffle (WT) | BT | WT | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| d RT I (min) | d RT II (s) | RT I (min) | RT II (s) | e Mean ± SD (µg·g−1) | Mean ± SD (µg·g−1) | ||||||
| Alcohols and Phenols | |||||||||||
| 1 | 1010 | 1016 | 2-Butanol | 78-92-2 | 925 | 7.09 | 1.39 | ND | ND | 0.032 ± 0.011 | ND |
| 2 | 1070 | 1078 | 2-Methyl-1-propanol | 78-83-1 | 883 | 9.26 | 1.43 | ND | ND | 0.266 ± 0.090 | ND |
| 3 | 1188 | 1206 | 2-Methyl-1-butanol | 137-32-6 | 879 | 14.56 | 1.64 | 14.63 | 1.63 | 1.695 ± 0.852 | 2.541 ± 0.235 |
| 4 | 1231 | 1241 | 1-Pentanol | 71-41-0 | 893 | 16.97 | 1.66 | 16.92 | 1.66 | 0.144 ± 0.028 | 0.069 ± 0.001 |
| 5 | 1333 | 1323 | 3-Methyl-1-pentanol | 589-35-5 | 872 | ND | ND | 22.81 | 1.88 | ND | 0.092 ± 0.010 |
| 6 | 1334 | 1345 | 1-Hexanol | 111-27-3 | 871 | 22.88 | 1.87 | ND | ND | 0.032 ± 0.007 | ND |
| 7 | 1410 | 1430 | 1-Methoxy-3-methyl benzene | 100-84-5 | 907 | 27.39 | 2.77 | ND | ND | 0.137 ± 0.058 | ND |
| 8 | 1431 | 1442 | 1-Octen-3-ol | 3391-86-4 | 893 | 28.59 | 2.22 | 28.49 | 2.24 | 0.218 ± 0.048 | 0.339 ± 0.035 |
| 9 | 1470 | 1481 | 2-Ethyl-1-hexanol | 104-76-7 | 881 | 30.92 | 2.40 | 30.91 | 2.40 | 0.082 ± 0.024 | 0.051 ± 0.004 |
| 10 | 1875 | 1875 | Phenylethyl Alcohol | 60-12-8 | 934 | 53.19 | 1.65 | 53.16 | 1.64 | 3.100 ± 1.264 | 1.956 ± 0.215 |
| 11 | 1880 | 1902 | Butylated Hydroxytoluene | 128-37-0 | 887 | 53.43 | 5.21 | 53.36 | 5.21 | 0.017 ± 0.001 | 0.099 ± 0.041 |
| 12 | 1950 | 1950 | β-Ethylphenethyl alcohol | 2035-94-1 | 811 | ND | ND | 57.51 | 2.15 | ND | 0.024 ± 0.003 |
| 13 | 2057 | 2068 | 3-Methylphenol | 108-39-4 | 803 | 63.32 | 1.30 | ND | ND | 0.033 ± 0.012 | ND |
| 14 | 2106 | 2107 | 2-Phenoxyethanol | 122-99-6 | 777 | 65.61 | 1.49 | ND | ND | 0.017 ± 0.005 | ND |
| 15 | 2286 | 2277 | 2,4-Di-tert-butylphenol | 96-76-4 | 914 | 72.49 | 1.85 | 72.39 | 1.86 | 0.255 ± 0.082 | 0.543 ± 0.175 |
| 16 | 2556 | 1779 | α-Methylbenzeneethanol | 698-87-3 | 780 | 80.61 | 1.34 | ND | ND | 0.121 ± 0.061 | ND |
| Aldehydes | |||||||||||
| 17 | 1017 | 1037 | (Z)-2-Butenal | 15798-64-8 | 883 | ND | ND | 7.33 | 1.54 | ND | 0.097 ± 0.021 |
| 18 | 1054 | 1051 | Hexanal | 66-25-1 | 906 | 8.77 | 2.54 | 8.70 | 2.53 | 0.716 ± 0.198 | 0.523 ± 0.005 |
| 19 | 1067 | 1088 | 2-Methyl-2-butenal | 1115-11-3 | 893 | 9.14 | 1.95 | ND | ND | 0.010 ± 0.004 | ND |
| 20 | 1105 | 1128 | (E)-2-Pentenal | 1576-87-0 | 861 | 10.58 | 1.93 | ND | ND | 0.004 ± 0.001 | ND |
| 21 | 1160 | 1184 | Heptanal | 111-71-7 | 865 | 13.24 | 3.30 | 13.09 | 3.33 | 0.033 ± 0.014 | 0.019 ± 0.004 |
| 22 | 1170 | 1200 | 3-Methyl-2-butenal | 107-86-8 | 791 | ND | ND | 13.74 | 1.92 | ND | 0.008 ± 0.001 |
| 23 | 1294 | 1319 | (Z)-2-Heptenal | 57266-86-1 | 912 | 20.59 | 2.90 | 20.51 | 2.91 | 0.099 ± 0.021 | 0.032 ± 0.003 |
| 24 | 1368 | 1387 | Nonanal | 124-19-6 | 837 | 24.81 | 4.71 | 24.70 | 4.73 | 0.047 ± 0.010 | 0.031 ± 0.008 |
| 25 | 1384 | 1404 | 5-Ethylcyclopent-1-enecarboxaldehyde | 36431-60-4 | 882 | 25.78 | 3.19 | 25.71 | 3.19 | 0.714 ± 0.225 a | 0.062 ± 0.004 b |
| 26 | 1400 | 1416 | (E)-2-Octenal | 2548-87-0 | 876 | 26.74 | 3.34 | 26.68 | 3.35 | 0.244 ± 0.074 | 0.061 ± 0.002 |
| 27 | 1486 | 1478 | Benzaldehyde | 100-52-7 | 896 | 31.81 | 1.93 | 31.76 | 1.94 | 0.223 ± 0.047 | 0.320 ± 0.014 |
| 28 | 1598 | 1622 | Benzeneacetaldehyde | 122-78-1 | 927 | 38.60 | 1.96 | 38.53 | 1.97 | 0.568 ± 0.119 | 0.551 ± 0.031 |
| 29 | 1682 | 1700 | Dodecanal | 112-54-9 | 861 | 42.90 | 5.97 | ND | ND | 0.200 ± 0.010 | ND |
| 30 | 1775 | 1767 | 2,4-Decadienal | 2363-88-4 | 837 | 47.89 | 3.10 | 47.84 | 3.10 | 0.024 ± 0.005 | 0.014 ± 0.001 |
| 31 | 1887 | 1907 | α-Ethylidene-benzeneacetaldehyde | 4411-89-6 | 852 | 53.80 | 2.35 | 53.76 | 2.35 | 0.076 ± 0.022 | 0.151 ± 0.016 |
| Hydrocarbons | |||||||||||
| 32 | 1003 | 1008 | α-Pinene | 80-56-8 | 913 | 6.84 | 6.44 | ND | ND | 0.040 ± 0.012 | ND |
| 33 | 2276 | 2322 | Fluorene | 86-73-7 | 824 | 72.05 | 2.35 | 72.02 | 2.35 | 0.011 ± 0.001 | 0.023 ± 0.017 |
| Ketones | |||||||||||
| 34 | — | 1012.6 | 2-Methyl-3-pentanone | 565-69-5 | 776 | ND | ND | 6.24 | 2.26 | ND | 0.005 ± 0.002 |
| 35 | 1000 | 1016 | 3-Methyl-2-pentanone | 565-61-7 | 852 | 6.72 | 2.24 | 6.73 | 2.24 | 0.014 ± 0.003 | 0.019 ± 0.007 |
| 36 | 1103 | 1108 | (E)-3-Penten-2-one | 3102-33-8 | 849 | 10.46 | 1.83 | ND | ND | 0.002 ± 0.000 | ND |
| 37 | 1158 | 1175 | 2-Heptanone | 110-43-0 | 867 | 13.12 | 3.14 | ND | ND | 0.007 ± 0.002 | ND |
| 38 | 1255 | 1271 | Acetoin | 513-86-0 | 836 | 18.30 | 1.42 | 18.37 | 1.42 | 0.292 ± 0.128 | 0.337 ± 0.078 |
| 39 | 1605 | 1607 | Acetophenone | 98-86-2 | 768 | 38.96 | 2.07 | ND | ND | 0.015 ± 0.001 | ND |
| 40 | 2431 | 2443 | Benzophenone | 119-61-9 | 772 | 76.99 | 1.99 | ND | ND | 0.014 ± 0.000 | ND |
| Acids | |||||||||||
| 41 | 1513 | 1508 | Propanoic acid | 79-09-4 | 935 | 33.46 | 1.17 | ND | ND | 0.654 ± 0.304 | ND |
| 42 | 1540 | 1544 | 2-Methylpropanoic acid | 79-31-2 | 939 | 35.10 | 1.33 | 35.22 | 1.20 | 3.808 ± 1.026 | 0.155 ± 0.021 |
| 43 | 1598 | 1613 | Butanoic acid | 107-92-6 | 874 | 38.60 | 1.22 | ND | ND | 1.005 ± 0.381 | ND |
| 44 | 1643 | 1647 | 3-Methyl-Butanoic acid | 503-74-2 | 835 | 40.89 | 1.37 | 40.83 | 1.32 | 11.014 ± 4.253 | 10.281 ± 1.507 |
| 45 | 1771 | 1776 | 3-Methyl-2-butenoic acid | 541-47-9 | 861 | ND | ND | 47.60 | 1.22 | ND | 0.008 ± 0.001 |
| 46 | 1780 | 1803 | 4-Methylpentanoic acid | 646-07-1 | 819 | ND | ND | 48.08 | 1.29 | ND | 0.013 ± 0.002 |
| 47 | 1821 | 1816 | Hexanoic acid | 142-62-1 | 909 | 50.30 | 1.37 | 50.26 | 1.33 | 5.247 ± 1.878 | 3.109 ± 0.143 |
| 48 | 1929 | 1934 | Heptanoic acid | 111-14-8 | 879 | 56.25 | 1.46 | 56.18 | 1.45 | 0.190 ± 0.046 a | 0.042 ± 0.006 b |
| 49 | 2041 | 2038 | Octanoic acid | 124-07-2 | 877 | 62.52 | 1.44 | 62.35 | 1.45 | 0.086 ± 0.003 a | 0.268 ± 0.011 b |
| 50 | 2150 | 2144 | Nonanoic acid | 112-05-0 | 847 | 67.30 | 1.45 | 67.19 | 1.46 | 0.032 ± 0.007 a | 0.075 ± 0.008 b |
| 51 | 2166 | 2182 | (E)-2-Octenoic acid | 1871-67-6 | 868 | 68.03 | 1.34 | 67.91 | 1.34 | 0.037 ± 0.011 | 0.049 ± 0.002 |
| 52 | 2544 | 2543 | Benzeneacetic acid | 103-82-2 | 895 | 80.25 | 1.21 | 80.25 | 1.20 | 0.891 ± 0.258 | 0.628 ± 0.036 |
| Esters | |||||||||||
| 53 | 1550 | 1550 | Isobornyl acetate | 125-12-2 | 798 | 35.67 | 5.76 | ND | ND | 0.019 ± 0.011 | ND |
| 54 | 2235 | 2241 | Hexadecanoic acid ethyl ester | 628-97-7 | 842 | 70.60 | 7.13 | ND | ND | 0.031 ± 0.005 | ND |
| 55 | 2453 | 2476 | (E)-9-Octadecenoic acid ethyl ester | 6114-18-7 | 897 | 77.67 | 6.09 | ND | ND | 0.400 ± 0.106 | ND |
| 56 | 2502 | 2515 | 9,12-Octadecadienoic acid ethyl ester | 7619-08-1 | 904 | 79.04 | 5.33 | ND | ND | 0.710 ± 0.174 | ND |
| 57 | 2502 | 2510 | Linoleic acid ethyl ester | 544-35-4 | 801 | 79.08 | 6.53 | 78.92 | 5.32 | 0.037 ± 0.016 | 0.051 ± 0.029 |
| 58 | 2581 | 2607 | Benzyl benzoate | 120-51-4 | 914 | 81.33 | 2.06 | 81.33 | 2.07 | 0.184 ± 0.042 | 1.373 ± 0.474 |
| 59 | 1966 | 1978 | Dehydromevalonic lactone | 2381-87-5 | 891 | ND | ND | 58.52 | 1.70 | ND | 0.113 ± 0.032 |
| Furans and Furanones | |||||||||||
| 60 | 1209 | 1215 | 2-Pentylfuran | 3777-69-3 | 915 | 15.65 | 4.75 | 15.51 | 4.80 | 0.020 ± 0.004 | 0.013 ± 0.001 |
| 61 | 1566 | 1589 | Dihydro-5-methyl-2(3H)furanone | 108-29-2 | 780 | 36.67 | 1.63 | ND | ND | 0.041 ± 0.011 | ND |
| 62 | 1711 | 1712 | 2(5H)furanone | 497-23-4 | 861 | 44.39 | 1.34 | 44.33 | 1.34 | 0.020 ± 0.007 | 0.124 ± 0.032 |
| 63 | 1960 | 1984 | Furyl hydroxymethyl ketone | 17678-19-2 | 730 | ND | ND | 58.12 | 1.49 | ND | 0.013 ± 0.001 |
| 64 | 1985 | 2003 | Dihydro-5-pentyl-2(3H)furanone | 104-61-0 | 846 | 59.58 | 2.53 | 59.57 | 2.53 | 0.014 ± 0.004 | 0.029 ± 0.004 |
| 65 | 2203 | 2270 | Dibenzofuran | 132-64-9 | 801 | 69.47 | 2.30 | ND | ND | 0.015 ± 0.003 | ND |
| Sulfur | |||||||||||
| 66 | 1047 | 1039 | Dimethyl disulfide | 624-92-0 | 751 | 8.41 | 2.05 | ND | ND | 0.006 ± 0.003 | ND |
| 67 | 1421 | 1429 | Methional | 3268-49-3 | 897 | ND | ND | 28.01 | 1.81 | ND | 0.900 ± 0.076 |
| 68 | 1650 | 1684 | (Methylthio)-cyclohexane | 7133-37-1 | 705 | ND | ND | 41.07 | 2.18 | ND | 0.609 ± 0.069 |
| 69 | 1691 | 1710 | 3-Methylthio-1-propanol | 505-10-2 | 906 | ND | ND | 43.24 | 1.50 | ND | 1.310 ± 0.249 |
| 70 | 2281 | 2298 | 3-(Methylthio)propanoic acid | 646-01-5 | 817 | 72.29 | 1.16 | 72.14 | 1.16 | 0.034 ± 0.011 a | 0.068 ± 0.002 b |
| 71 | 1906 | 1936 | Benzothiazole | 95-16-9 | 701 | ND | ND | 54.85 | 2.15 | ND | 0.007 ± 0.002 |
| No. a | Compound | Aroma Threshold Values | Description |
|---|---|---|---|
| 1 | 2-Butanol | 1700 ppb b | — |
| 2 | 2-Methyl-1-propanol | 360 ppb to 3.3 ppm c | A penetrating, wine-like, disagreeable odor c |
| 5 | 3-Methyl-1-pentanol | 830 ppb to 1.2 ppm c | A fruity, green, slightly pungent odor c |
| 6 | 1-Hexanol | 200 ppb to 2.5 ppm c | An herbaceous, woody, fragrant, mild, sweet, green, fruity odor c |
| 7 | 1-Methoxy-3-methyl benzene | d | — |
| 12 | β-Ethylphenethyl alcohol | — | — |
| 13 | 3-Methylphenol | 650 ppb b | A dry, tarry, medicinal-leathery odor c |
| 14 | 2-Phenoxyethanol | — | — |
| 16 | α-Methylbenzeneethanol | — | — |
| 17 | (Z)-2-Butenal | — | — |
| 19 | 2-Methyl-2-butenal | — | — |
| 20 | (E)-2-Pentenal | — | fruity, strawberry e |
| 22 | 3-Methyl-2-butenal | — | an almond odor c |
| 25 | 5-Ethylcyclopent-1-enecarboxaldehyde | — | — |
| 29 | Dodecanal | 0.5 to 1.5 ppb c | A characteristic fatty odor reminiscent of violet on dilution c |
| 32 | α-Pinene | 2.5 to 62 ppb c | A characteristic odor of pine; it is turpentine-like c |
| 34 | 2-Methyl-3-pentanone | — | — |
| 36 | (E)-3-Penten-2-one | — | — |
| 37 | 2-Heptanone | 1 ppb to 1.33 ppm c | A fruity, spicy, cinnamon, banana, slightly spicy odor c |
| 39 | Acetophenone | 170 ppb c | A characteristic sweet, pungent and strong medicinal odor c |
| 40 | Benzophenone | — | A delicate, persistent, rose-like odor c |
| 41 | Propanoic acid | 5 to 10 ppm c | A pungent, rancid odor c |
| 43 | Butanoic acid | 240 ppb to 4.8 ppm c | A persistent, penetrating, rancid, butter-like odor c |
| 45 | 3-Methyl-2-butenoic acid | — | A green, phenolic, dairy aroma c |
| 46 | 4-Methylpentanoic acid | 810 ppb c | an unpleasant, sour, penetrating odor c |
| 48 | Heptanoic acid | 640 ppb to 10.4 ppm c | A disagreeable rancid, sour, sweat-like, fatty odor c |
| 49 | Octanoic acid | 910 ppb to 19 ppm c | A mildly unpleasant odor c |
| 50 | Nonanoic acid | 3 to 9 ppm c | A fatty, characteristic odor c |
| 53 | Isobornyl acetate | — | A pleasant, camphor-like odor reminiscent of some varieties of pine needles c |
| 54 | Hexadecanoic acid ethyl ester | 2 ppm c | A mild, waxy sweet odor c |
| 55 | (E)-9-Octadecenoic acid ethyl ester | — | — |
| 56 | 9,12-Octadecadienoic acid ethyl ester | — | — |
| 59 | Dehydromevalonic lactone | — | — |
| 61 | Dihydro-5-methyl-2(3H)furanone | — | A sweet, herbaceous odor c |
| 63 | Furyl hydroxymethyl ketone | — | — |
| 65 | Dibenzofuran | — | Rotten, rubber, fat, moss e |
| 66 | Disulfide dimethyl | 0.16 to 1.2 ppb c | A diffuse, intense onion odor c |
| 67 | Methional | 0.02 ppb c | A powerful, onion, meat-like odor |
| 68 | (Methylthio)-cyclohexane | — | — |
| 69 | 3-Methylthio-1-propanol | 0.2 ppb c | A powerful, sweet, soup or meat-like odor and flavor in high dilution c |
| 70 | 3-(Methylthio)propanoic acid | — | — |
| 71 | Benzothiazole | 80 to 450 ppb c | A delicate, persistent, rose-like odor similar to that of quinoline c |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Chen, H.; Sun, B.; Mao, X.; Zhang, Y.; Zhou, Y. Comparative Analysis of Volatile Composition in Chinese Truffles via GC × GC/HR-TOF/MS and Electronic Nose. Int. J. Mol. Sci. 2016, 17, 412. https://doi.org/10.3390/ijms17040412
Zhang N, Chen H, Sun B, Mao X, Zhang Y, Zhou Y. Comparative Analysis of Volatile Composition in Chinese Truffles via GC × GC/HR-TOF/MS and Electronic Nose. International Journal of Molecular Sciences. 2016; 17(4):412. https://doi.org/10.3390/ijms17040412
Chicago/Turabian StyleZhang, Ning, Haitao Chen, Baoguo Sun, Xueying Mao, Yuyu Zhang, and Ying Zhou. 2016. "Comparative Analysis of Volatile Composition in Chinese Truffles via GC × GC/HR-TOF/MS and Electronic Nose" International Journal of Molecular Sciences 17, no. 4: 412. https://doi.org/10.3390/ijms17040412
APA StyleZhang, N., Chen, H., Sun, B., Mao, X., Zhang, Y., & Zhou, Y. (2016). Comparative Analysis of Volatile Composition in Chinese Truffles via GC × GC/HR-TOF/MS and Electronic Nose. International Journal of Molecular Sciences, 17(4), 412. https://doi.org/10.3390/ijms17040412