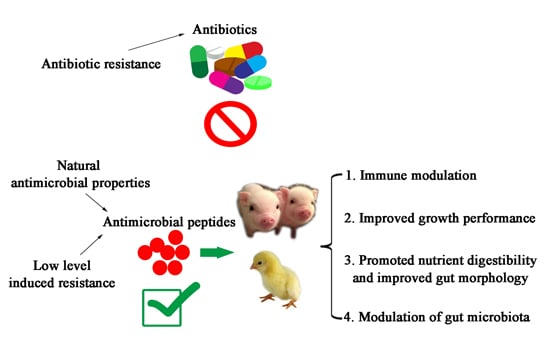
Antimicrobial Peptides as Potential Alternatives to Antibiotics in Food Animal Industry
Abstract
:1. Introduction
2. Antimicrobial Peptides
3. Structure of Antimicrobial Peptides
4. Broad-Spectrum Activity
4.1. Antibacterial Activity
4.2. Antifungal Activity
4.3. Antiviral Activity
5. Low Level Induced Resistance to AMPs
6. Immune Modulation
7. Application in Non-Ruminant Nutrition
7.1. Improved Growth Performance
7.2. Impact on Nutrient Digestibility and Gut Morphology
7.3. Modulation of Gut Microbiota
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cromwell, G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002, 13, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial peptides: Tackling of a crisis for the health and wealth of nations. Available online: http://amr-review.org/sites/fefault/files/AMR%20Review%20paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on December 2014).
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Sharma, B.K.; Raghava, G.P. Analysis and prediction of antibacterial peptides. BMC Bioinform. 2007, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.P. Cationic antimicrobial peptides-Issues for potential clinical use. Biodrugs 2003, 17, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Sahl, H. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 2006, 4, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Thacker, P.A.; Watford, M.; Qiao, S. Functions of Antimicrobial Peptides in Gut Homeostasis. Curr. Protein Pept. Sci. 2015, 16, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E. Cationic peptides: Effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001, 1, 156–164. [Google Scholar] [CrossRef]
- Zhang, L.J.; Parente, J.; Harris, S.A.; Woods, D.E.; Hancock, R.; Fallal, T.J. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob. Agents Chemother. 2005, 49, 2921–2927. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.P.; Hancock, R.E. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta—Biomembr. 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Yang, L.; Harroun, T.A.; Weiss, T.M.; Ding, L.; Huang, H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001, 81, 1475–1485. [Google Scholar] [CrossRef]
- Hoffmann, J.A. Innate immunity of insects. Curr. Opin. Immunol. 1995, 7, 4–10. [Google Scholar] [CrossRef]
- Andreu, D.; Merrifield, R.B.; Steiner, H.; Boman, H.G. N-terminal analogues of cecropin A: Synthesis, antibacterial activity, and conformational properties. Biochemistry 1985, 24, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, L.; Weiser, J.N.; Axelsen, P.H. Antibacterial and antimembrane activities of cecropin A in Escherichia coli. Antimicrob. Agents Chemother. 2000, 44, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A.; Hetru, C. Insect defensins: Inducible antibacterial peptides. Immunol. Today 1992, 13, 411–415. [Google Scholar] [CrossRef]
- Simmaco, M.; Mignogna, G.; Barra, D. Antimicrobial peptides from amphibian skin: What do they tell us? Biopolymers 1998, 47, 435–450. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Lee, W.H.; Yang, X.; Zhang, Y. Novel peptides from skins of amphibians showed broad-spectrum antimicrobial activities. Chem. Biol. Drug Des. 2016, 87, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hu, N.; Lyu, P.; Ma, J.; Wang, L.; Zhou, M.; Guo, S.; Chen, T.; Shaw, C. Hylaranins: Prototypes of a new class of amphibian antimicrobial peptide from the skin secretion of the oriental broad-folded frog, Hylarana latouchii. Amino Acids 2014, 46, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Seebah, S.; Low, S.M.; Zhou, L.; Liu, S.P.; Li, J.; Beuerman, R.W. Defensins: Antimicrobial peptides for therapeutic development. Biotechnol. J. 2007, 2, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2001, 276, 5707–5713. [Google Scholar] [CrossRef] [PubMed]
- Bals, R.; Wang, X.; Zasloff, M.; Wilson, J.M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 1998, 95, 9541–9546. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.N.; Bishop, B.M.; van Hoek, M.L. Susceptibility of pseudomonas aeruginosa biofilm to α-helical peptides: D-enantiomer of LL-37. Front. Microbiol. 2011, 2, 128. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Cho, Y.; Dinh, N.N.; Waring, A.J.; Lehrer, R.I. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 1998, 42, 2206–2214. [Google Scholar] [PubMed]
- Li, Y.; Xiang, Q.; Zhang, Q.; Huang, Y.; Su, Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 2012, 37, 207–215. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, A.J.; Walsh, T.J. Antifungal peptides: Novel therapeutic compounds against emerging pathogens. Antimicrob. Agents Chemother. 1999, 43, 1–11. [Google Scholar] [PubMed]
- Benincasa, M.; Scocchi, M.; Pacor, S.; Tossi, A.; Nobili, D.; Basaglia, G.; Busetti, M.; Gennaro, R. Fungicidal activity of five cathelicidin peptides against clinically isolated yeasts. J. Antimicrob. Chemother. 2006, 58, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S.; Nayyar, N.; Li, W.; Edgerton, M. Human β-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob. Agents Chemother. 2007, 51, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.W.; Yang, C.Y.; Chang, H.T.; Lan, C.Y. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS ONE 2011, 6, e17755. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.I.; Noll, K.S.; Xu, S.; Li, J.; Huang, Q.; Sinko, P.J.; Wachsman, M.B.; Chikindas, M.L. Safety, formulation, and in vitro antiviral activity of the antimicrobial peptide subtilosin against herpes simplex virus type 1. Probiotics Antimicrob. Proteins 2013, 5, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J.; Donis, R.O. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE 2011, 6, e25333. [Google Scholar] [CrossRef] [PubMed]
- Lorin, C.; Saidi, H.; Belaid, A.; Zairi, A.; Baleux, F.; Hocini, H.; Bélec, L.; Hani, K.; Tangy, F. The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology 2005, 334, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Tecle, T.; Verma, A.; Crouch, E.; White, M.; Hartshorn, K.L. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J. Gen. Virol. 2013, 94, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Dubyak, G.R.; Lederman, M.M.; Weinberg, A. Cutting edge: Human β defensin 3–a novel antagonist of the HIV-1 coreceptor CXCR4. J. Immunol. 2006, 177, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.A.; Wang, W.; Rawat, S.S.; Jung, G.; Waring, A.J.; Cole, A.M.; Lu, H.; Yan, X.; Daly, N.L.; Craik, D.J.; et al. θ-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J. Biol. Chem. 2006, 281, 18787–18792. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; Siriwardena, T.N.; Stach, M.; Tinguely, R.; Kasraian, S.; Luzzaro, F.; Leib, S.L.; Darbre, T.; Reymond, J.L.; Endimiani, A. In vitro activity of the novel antimicrobial peptide dendrimer G3KL against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2015, 59, 7915–7918. [Google Scholar] [CrossRef] [PubMed]
- Rajamuthiah, R.; Jayamani, E.; Conery, A.L.; Fuchs, B.B.; Kim, W.; Johnston, T.; Vilcinskas, A.; Ausubel, F.M.; Mylonakis, E. A Defensin from the model beetle tribolium castaneum acts synergistically with telavancin and daptomycin against multidrug resistant Staphylococcus aureus. PLoS ONE 2015, 10, e128576. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Sambanthamoorthy, K.; Palys, T.; Paranavitana, C. The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter baumannii. Peptides 2013, 49, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Gao, B. A fossil antibacterial peptide gives clues to structural diversity of cathelicidin-derived host defense peptides. FASEB J. 2009, 23, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Pag, U.; Sahl, H.G. Multiple activities in lantibiotics–models for the design of novel antibiotics? Curr. Pharm. Des. 2002, 8, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.C.; Pena, O.M.; Hancock, R.E. Host defense peptides: Front-line immunomodulators. Trends Immunol. 2014, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.H.; Yuan, W.; Deng, H.D.; Deng, J.L.; Dan, Q.X.; Jin, H.T.; Tian, C.L.; Peng, X.; Liang, Z.; Gao, S.; et al. Effects of antibacterial peptide on cellular immunity in weaned piglets. J. Anim. Sci. 2015, 93, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Wang, Y.; Wang, Y.; Liu, J.; Xu, Z. Effect of dietary lactoferrin on the immune functions and serum iron level of weanling piglets. J. Anim. Sci. 2007, 85, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; She, R.; Wang, K.; Bao, H.; Zhang, Y.; Luo, D.; Hu, Y.; Ding, Y.; Wang, D.; Peng, K. Effects of rabbit sacculus rotundus antimicrobial peptides on the intestinal mucosal immunity in chickens. Poult. Sci. 2008, 87, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; She, R.; Liu, T.; Zhang, Y.; Peng, K.S.; Luo, D.; Yue, Z.; Ding, Y.; Hu, Y.; Liu, W.; et al. Effects of pig antibacterial peptides on growth performance and intestine mucosal immune of broiler chickens. Poult. Sci. 2009, 88, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, W.; She, R.; Sun, Q.; Liu, Y.; Hu, Y.; Liu, L.; Yang, Y.; Peng, K. Effects of swine gut antimicrobial peptides on the intestinal mucosal immunity in specific-pathogen-free chickens. Poult. Sci. 2009, 88, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Thacker, P.A. Alternatives to antibiotics as growth promoters for use in swine production: A review. J. Anim. Sci. Biotechnol. 2013, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Shao, F.; Wu, M.; Ren, W.; Xiong, X.; Tan, B.; Yin, Y. The application of antimicrobial peptides as growth and health promoters for swine. J. Anim. Sci. Biotechnol. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shan, T.; Xu, Z.; Liu, J.; Feng, J. Effect of lactoferrin on the growth performance, intestinal morphology, and expression of PR-39 and protegrin-1 genes in weaned piglets. J. Anim. Sci. 2006, 84, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yin, Y.; Zhang, Y.; Huang, R.; Sun, Z.; Li, T.; Chu, W.; Kong, X.; Li, L.; Geng, M.; et al. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin-lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br. J. Nutr. 2009, 101, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lee, S.H.; Park, Y.K.; Kwon, I.K.; Chae, B.J. Effects of dietary supplementation of antimicrobial peptide-A3 on growth performance, nutrient digestibility, intestinal and fecal microflora and intestinal morphology in weanling pigs. Anim. Feed Sci. Technol. 2012, 177, 98–107. [Google Scholar] [CrossRef]
- Yoon, J.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lohakare, J.; Park, Y.K.; Park, J.C.; Kwon, I.K.; Chae, B.J. Effects of dietary supplementation with antimicrobial peptide-P5 on growth performance, apparent total tract digestibility, faecal and intestinal microflora and intestinal morphology of weanling pigs. J. Sci. Food Agric. 2013, 93, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.C.; Ingale, S.L.; Kim, J.S.; Park, Y.K.; Kwon, I.K.; Chae, B.J. Effects of dietary supplementation with an antimicrobial peptide-P5 on growth performance, nutrient retention, excreta and intestinal microflora and intestinal morphology of broilers. Anim. Feed Sci. Technol. 2013, 85, 78–84. [Google Scholar] [CrossRef]
- Choi, S.C.; Ingale, S.L.; Kim, J.S.; Park, Y.K.; Kwon, I.K.; Chae, B.J. An antimicrobial peptide-A3: Effects on growth performance, nutrient retention, intestinal and faecal microflora and intestinal morphology of broilers. Br. Poult. Sci. 2013, 54, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shan, T.; Xu, Z.; Feng, J.; Wang, Z. Effects of the lactoferrin (LF) on the growth performance, intestinal microflora and morphology of weanling pigs. Anim. Feed Sci. Technol. 2007, 135, 263–272. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Xu, C.L.; An, Z.H.; Liu, J.X.; Feng, J. Effect of dietary bovine lactoferrin on performance and antioxidant status of piglets. Anim. Feed Sci. Technol. 2008, 140, 326–336. [Google Scholar] [CrossRef]
- Xiao, H.; Wu, M.M.; Tan, B.E.; Yin, Y.L.; Li, T.J.; Xiao, D.F.; Li, L. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity. J. Anim. Sci. 2013, 91, 4772–4780. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, F.; Huang, Z.; Liu, H.; Xie, C.; Zhang, J.; Thacker, P.A.; Qiao, S. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides 2012, 35, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.F.; He, J.G. Dose-response effects of an antimicrobial peptide, a cecropin hybrid, on growth performance, nutrient utilisation, bacterial counts in the digesta and intestinal morphology in broilers. Br. J. Nutr. 2012, 108, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yang, Y.X.; Choi, J.Y.; Shinde, P.L.; Yoon, S.Y.; Hahn, T.W.; Lim, H.T.; Park, Y.; Hahm, K.S.; Joo, J.W.; et al. Potato (Solanum tuberosum L. cv. Gogu valley) protein as a novel antimicrobial agent in weanling pigs. J. Anim. Sci. 2008, 86, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zeng, X.F.; Wang, Q.W.; Zhu, J.L.; Peng, Q.; Hou, C.L.; Thacker, P.; Qiao, S.Y. The antimicrobial peptide sublancin ameliorates necrotic enteritis induced by Clostridium perfringens in broilers. J. Anim. Sci. 2015, 93, 4750–4760. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Li, Y.; Chen, D.; Yu, B.; Chen, G.; Zheng, P.; Mao, X.; Yu, J.; He, J. Recombinant plectasin elicits similar improvement in the performance and intestinal mucosa growth and activity in weaned pigs as an antibiotic. Anim. Feed Sci. Technol. 2016, 211, 216–226. [Google Scholar] [CrossRef]
| Antimicrobial Peptide | Dose, mg/kg | Treatment Effects (%, Compared to Control) | References | |||
|---|---|---|---|---|---|---|
| Animal | ADG a | ADFI a | G:F a | |||
| Antimicrobial peptide-A3 | 60 | Weanling pigs | 2 | 1 | 0 | [58] |
| 90 | 5 | 2 | 5 | |||
| Antimicrobial peptide-P5 | 40 | Weanling pigs | 4 | 1 | 2 | [59] |
| 60 | 8 | 3 | 5 | |||
| Lactoferrin | 1000 | Weanling pigs | 34 | 17 | 15 | [62] |
| 1000 | 42 | 21 | 17 | [56] | ||
| Bovine lactoferrin | 1250 | Weanling pigs | 16 | 15 | 0 | [63] |
| 2500 | 13 | 13 | 0 | |||
| Bovine lactoferrin-lactoferrampin | 100 | Weanling pigs | 24 | 17 | 6 | [57] |
| Composite antimicrobial peptides | 4000 | Weanling pigs | −6 | −17 | 15 | [64] |
| Cecropin AD | 400 | Weanling pigs | 4 | 1 | 3 | [65] |
| Antimicrobial peptide-A3 | 60 | Broilers | 1 | 0 | 1 | [61] |
| 90 | 4 | 2 | 2 | |||
| Antimicrobial peptide-P5 | 40 | Broilers | 4 | 2 | 2 | [60] |
| 60 | 7 | 3 | 4 | |||
| Pig antimicrobial peptide | 150 | Broilers | 19 | 2 | 17 | [52] |
| 200 | 20 | 1 | 18 | |||
| Cecropin A-D-Asn | 2 | Broilers | 0 | −5 | 5 | [66] |
| 4 | 2 | −6 | 9 | |||
| 6 | 1 | −16 | 20 | |||
| 8 | -2 | −14 | 14 | |||
| Item | Uninfected Control | Infected Control | Sublancin (mg/L of Water) | Lincomycin (mg/L of Water) | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| 2.88 | 5.76 | 11.52 | 75 | |||||
| Duodenum | ||||||||
| Villus height, μm | 910.4 b | 880.2 b | 906.6 b | 1016.3 a,b | 1104.0 a | 1144.0 a | 34.61 | <0.01 |
| Crypt depth, μm | 188.7 | 197.1 | 177.8 | 192.4 | 186.8 | 179.2 | 6.72 | 0.35 |
| Villus height:crypt depth | 4.85 b,c | 4.46 c | 5.14 b,c | 5.29 b,c | 5.92 a,b | 6.44 a | 0.24 | <0.01 |
| Jejunum | ||||||||
| Villus height, μm | 805.2 | 776.4 | 873.6 | 903.2 | 918.8 | 927.5 | 35.96 | 0.07 |
| Crypt depth, μm | 159.1 | 180.1 | 146.5 | 168.7 | 174.8 | 158.4 | 8.62 | 0.14 |
| Villus height:crypt depth | 5.14 a,b | 4.32 b | 6.11 a | 5.45 a,b | 5.26 a,b | 5.88 a | 0.35 | 0.03 |
| Ileum | ||||||||
| Villus height, μm | 588.0 | 576.0 | 608.7 | 624.1 | 651.4 | 544.6 | 36.16 | 0.41 |
| Crypt depth, μm | 134.9 a,b | 146.7 a | 130.4 a,b | 136.1 a,b | 123.6 a,b | 100.4 b | 8.64 | 0.02 |
| Villus height:crypt depth | 4.40 | 3.97 | 4.79 | 4.74 | 5.30 | 5.55 | 0.40 | 0.14 |
| Antimicrobial Peptide | Animal | Treatment Effects | References |
|---|---|---|---|
| Lactoferrin | Weanling pigs | Reduced total viable counts of E. coli and Salmonella in the small intestine | [62] |
| Bovine lactoferrin-lactoferrampin | Weanling pigs | Decreased the counts of E. coli in the ileum, caecum and colon and increased the counts of Lactobacilli and Bifidobacteria in the ileum, caecum and colon | [57] |
| Antimicrobial peptide-A3 | Weanling pigs | Reduced total anaerobic bacteria, coliforms and Clostridium spp. in the ileum, cecum and feces | [58] |
| Antimicrobial peptide-P5 | Weanling pigs | Reduced fecal and intestinal coliforms and caecal Clostridium spp. | [59] |
| Potato protein | Weanling pigs | Decreased viable counts of total bacteria, coliforms and Staphylococcus spp. in caecum and rectum | [67] |
| Cecropin AD | Weanling pigs | Decreased total aerobes while increasing total anaerobes in the ileum and increased the numbers of Lactobacillus in the cecum | [65] |
| Recombinant plectasin | Weanling pigs | Increased the abundance of Bifidobacterium in the ileum | [69] |
| Cecropin A-D-Asn | Broilers | Decreased aerobic bacteria counts in both jejunal and caecal digesta | [66] |
| Antimicrobial peptide-A3 | Broilers | Reduced coliforms and Clostridium spp. counts in feces | [61] |
| Antimicrobial peptide-P5 | Broilers | Reduced excreta total anaerobic bacteria and coliforms | [60] |
| Sublancin | Broilers | Reduced Clostridium perfringens in the cecum | [68] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zeng, X.; Yang, Q.; Qiao, S. Antimicrobial Peptides as Potential Alternatives to Antibiotics in Food Animal Industry. Int. J. Mol. Sci. 2016, 17, 603. https://doi.org/10.3390/ijms17050603
Wang S, Zeng X, Yang Q, Qiao S. Antimicrobial Peptides as Potential Alternatives to Antibiotics in Food Animal Industry. International Journal of Molecular Sciences. 2016; 17(5):603. https://doi.org/10.3390/ijms17050603
Chicago/Turabian StyleWang, Shuai, Xiangfang Zeng, Qing Yang, and Shiyan Qiao. 2016. "Antimicrobial Peptides as Potential Alternatives to Antibiotics in Food Animal Industry" International Journal of Molecular Sciences 17, no. 5: 603. https://doi.org/10.3390/ijms17050603
APA StyleWang, S., Zeng, X., Yang, Q., & Qiao, S. (2016). Antimicrobial Peptides as Potential Alternatives to Antibiotics in Food Animal Industry. International Journal of Molecular Sciences, 17(5), 603. https://doi.org/10.3390/ijms17050603