Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD—A Systematic Review
Abstract
:1. Introduction
2. Definitions of Normal Liver Fat
2.1. Biochemical and Histologic Definitions
2.2. Proton Magnetic Resonance Spectroscopy (1H-MRS)
2.3. Magnetic Resonance Imaging (MRI)
2.4. Ultrasound (US)
2.5. Computed Tomography (CT)
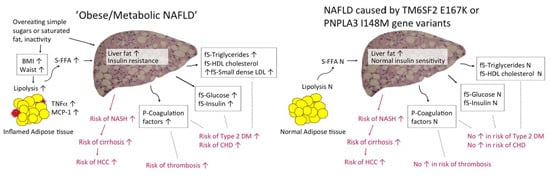
3. Non-Alcoholic Fatty Liver Disease (NAFLD) and Insulin Sensitivity
3.1. Insulin Resistance in “Obese/Metabolic NAFLD”
3.2. “Patatin-Like Phospholipase Domain-Containing 3 (PNPLA3) NAFLD” and Insulin Sensitivity
3.3. “Transmembrane 6 Superfamily Member 2 (TM6SF2) NAFLD” and Insulin Sensitivity
4. Materials and Methods
5. Conclusions
Future Research and Uncertainties
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 1H-MRS | proton magnetic resonance spectroscopy |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| BMI-SDS | body mass index standard deviation score |
| CHD | coronary heart disease |
| CoA | coenzyme A |
| CT | computed tomography |
| DM | diabetes mellitus |
| DHS | Dallas Heart Study |
| FFA | free fatty acids |
| fS | fasting serum |
| HDL | high density lipoprotein |
| MCP-1 | monocyte chemoattractant protein-1 |
| HCC | hepatocellular carcinoma |
| HDL | high density lipoprotein |
| HOMA-IR | homeostasis model assessment for insulin resistance |
| LDL | low density lipoprotein |
| MetS | metabolic syndrome |
| MRI | magnetic resonance imaging |
| NAFL | non-alcoholic fatty liver |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| OGTT | oral glucose tolerance test |
| P | plasma |
| PDFF | proton density fat fraction |
| PNPLA3 | patatin-like phospholipase domain-containing 3 |
| TM6SF2 | transmembrane 6 superfamily member 2 |
| TNF-α | tumor necrosis factor-α |
| US | ultrasound |
| VLDL | very low density lipoprotein |
References
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am. J. Gastroenterol. 2012, 107, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology 2010, 52, 774–788. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A. The NAS and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015, 149, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Hagstrom, H.; Nasr, P.; Fredrikson, M.; Stal, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Hardy, T.; Henderson, E.; Burt, A.D.; Day, C.P.; Anstee, Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 2015, 62, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Charlotte, F.; Fedchuk, L.; Bedossa, P.; Lebray, P.; Poynard, T.; Ratziu, V.; LIDO Study Group. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J. Hepatol. 2013, 59, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Wong, G.L.-H.; Choi, P.C.-L.; Chan, A.W.-H.; Li, M.K.-P.; Chan, H.-Y.; Chim, A.M.-L.; Yu, J.; Sung, J.J.-Y.; Chan, H.L.-Y. Disease progression of non-alcoholic fatty liver disease: A prospective study with paired liver biopsies at 3 years. Gut 2010, 59, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Conca, P.; Riccio, A.; Tarantino, M.; di Minno, M.N.; Chianese, D.; Pasanisi, F.; Contaldo, F.; Scopacasa, F.; Capone, D. Enhanced serum concentrations of transforming growth factor-beta1 in simple fatty liver: Is it really benign? J. Transl. Med. 2008, 6. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Castaño, G.O.; Scian, R.; Mallardi, P.; Fernández Gianotti, T.; Burgueño, A.L.; San Martino, J.; Pirola, C.J. Genetic variation in transmembrane 6 superfamily member 2 and the risk of nonalcoholic fatty liver disease and histological disease severity. Hepatology 2015, 61, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, P.K.; Zhou, Y.; Sädevirta, S.; Leivonen, M.; Arola, J.; Orešič, M.; Hyötyläinen, T.; Yki-Järvinen, H. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Donhoffer, H. Quantitative estimation of lipids in needle biopsy sized specimens of cadaver liver. Acta Med. Acad. Sci. Hung 1974, 31, 47–49. [Google Scholar] [PubMed]
- Hoyumpa, D.A.M., Jr.; Greene, H.L.; Dunn, G.D.; Schenker, S. Fatty liver: Biochemical and clinical considerations. Dig. Dis. Sci. 1975, 20, 1142–1170. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Nonalcoholic steatohepatitis clinical research network design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Tiniakos, D.G. Histopathology of nonalcoholic fatty liver disease. World J. Gastroenterol. 2010, 16, 5286–5296. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Poitou, C.; Veyrie, N.; Bouillot, J.-L.; Basdevant, A.; Paradis, V.; Tordjman, J.; Clément, K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012, 56, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Korenblat, K.M.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 2008, 134, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Laurell, S.; Lundquist, A. Lipid composition of human liver biopsy specimens. Acta Med. Scand. 1971, 189, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Piekarski, J.; Goldberg, H.I.; Royal, S.A.; Axel, L.; Moss, A.A. Difference between liver and spleen CT numbers in the normal adult: Its usefulness in predicting the presence of diffuse liver disease. Radiology 1980, 137, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E462–E468. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Dufour, S.; Feng, J.; Befroy, D.; Dziura, J.; Dalla Man, C.; Cobelli, C.; Shulman, G.I. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc. Natl. Acad. Sci. USA 2006, 103, 18273–18277. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, M.H.; Gardner, K.G.; Potter, C.J.; Schmalbrock, P.; Smith, M.A. Introduction of fast MR imaging in the assessment of hepatic steatosis. Magn. Reson. Imaging 1997, 15, 287–293. [Google Scholar] [CrossRef]
- Joseph, A.E.A.; Dewbury, K.C.; McGuire, P.G. Ultrasound in the detection of chronic liver disease (the “bright liver”). Br. J. Radiol. 1978, 52, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Saverymuttu, S.H.; Joseph, A.E.; Maxwell, J.D. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br. Med. J. (Clin. Res. Ed.) 1986, 292, 13–15. [Google Scholar] [CrossRef]
- Bohte, A.E.; van Werven, J.R.; Bipat, S.; Stoker, J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: A meta-analysis. Eur. Radiol. 2011, 21, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Ricci, C.; Masutti, F.; Vidimari, R.; Crocé, L.S.; Bercich, L.; Tiribelli, C.; Dalla Palma, L. Fatty infiltration of the liver: Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Investig. Radiol. 1993, 28, 297–302. [Google Scholar] [CrossRef]
- Szczepaniak, L.S.; Babcock, E.E.; Schick, F.; Dobbins, R.L.; Garg, A.; Burns, D.K.; McGarry, J.D.; Stein, D.T. Measurement of intracellular triglyceride stores by H spectroscopy: Validation in vivo. Am. J. Physiol. Endocrinol. Metab. 1999, 276, E977–E989. [Google Scholar]
- Kotronen, A.; Vehkavaara, S.; Seppälä-Lindroos, A.; Bergholm, R.; Yki-Järvinen, H. Effect of liver fat on insulin clearance. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1709–E1715. [Google Scholar] [CrossRef] [PubMed]
- Cowin, G.J.; Jonsson, J.R.; Bauer, J.D.; Ash, S.; Ali, A.; Osland, E.J.; Purdie, D.M.; Clouston, A.D.; Powell, E.E.; Galloway, G.J. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J. Magn. Reson. Imaging 2008, 28, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Lam, J.; Peterson, M.R.; Middleton, M.; Hamilton, G.; Le, T.-A.; Bettencourt, R.; Changchien, C.; Brenner, D.A.; Sirlin, C.; et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013, 58, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, C.; Becker, U.; Winkler, K.; Christoffersen, P.; Jensen, M.; Henriksen, O. Quantification of liver fat using magnetic resonance spectroscopy. Magn. Reson. Imaging 1994, 12, 487–495. [Google Scholar] [CrossRef]
- Dixon, W.T. Simple proton spectroscopic imaging. Radiology 1984, 153, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Rofsky, N.M.; Weinreb, J.C.; Ambrosino, M.M.; Safir, J.; Krinsky, G. Comparison between in-phase and opposed-phase T1-weighted breath-hold FLASH sequences for hepatic imaging. J. Comput. Assist. Tomogr. 1996, 20, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, F.H.; Yokoo, T.; Aganovic, L.; Hanna, R.F.; Bydder, M.; Middleton, M.S.; Hamilton, G.; Chavez, A.D.; Schwimmer, J.B.; Sirlin, C.B. Fatty liver disease: MR Imaging techniques for the detection and quantification of liver steatosis1. Radiographics 2009, 29, 231–260. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.K.; Chenevert, T.L.; Londy, F.J.; Gulani, V.; Swanson, S.D.; McKenna, B.J.; Appelman, H.D.; Adusumilli, S.; Greenson, J.K.; Conjeevaram, H.S. Hepatic fat fraction: MR imaging for quantitative measurement and display—Early experience 1. Radiology 2005, 237, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, M.H.; Stevens, W.R. Rapid MRI using a modified Dixon technique: A non-invasive and effective method for detection and monitoring of fatty metamorphosis of the liver. Pediatr. Radiol. 2001, 31, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Hines, C.D.G.; Frydrychowicz, A.; Hamilton, G.; Tudorascu, D.L.; Vigen, K.K.; Yu, H.; McKenzie, C.A.; Sirlin, C.B.; Brittain, J.H.; Reeder, S.B. T1 independent, T2* corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J. Magn. Reson. Imaging 2011, 33, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Meisamy, S.; Hines, C.D.G.; Hamilton, G.; Sirlin, C.B.; McKenzie, C.A.; Yu, H.; Brittain, J.H.; Reeder, S.B. Quantification of hepatic steatosis with T1-independent, T2*-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 2011, 258, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.H.; Cruite, I.; Shiehmorteza, M.; Wolfson, T.; Gamst, A.C.; Hamilton, G.; Bydder, M.; Middleton, M.S.; Sirlin, C.B. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J. Magn. Reson. Imaging 2011, 34, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Permutt, Z.; Le, T.A.; Peterson, M.R.; Seki, E.; Brenner, D.A.; Sirlin, C.; Loomba, R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease—MRI accurately quantifies hepatic steatosis in NAFLD. Aliment. Pharmacol. Ther. 2012, 36, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Peterson, M.R.; Brenner, D.A.; Heba, E.; Sirlin, C.; Loomba, R. Association between novel MRI-estimated pancreatic fat and liver histology-determined steatosis and fibrosis in non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2013, 37, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Idilman, I.S.; Aniktar, H.; Idilman, R.; Kabacam, G.; Savas, B. Hepatic steatosis: Quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 2013, 267, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Tan, J.; Sun, M.; Hamilton, G.; Bydder, M.; Wolfson, T.; Gamst, A.C.; Middleton, M.; Brunt, E.M.; Loomba, R.; et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013, 267, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Needleman, L.; Kurtz, A.B.; Rifkin, M.D.; Cooper, H.S.; Pasto, M.E.; Goldberg, B.B. Sonography of diffuse benign liver-disease—Accuracy of pattern-recognition and grading. Am. J. Roentgenol. 1986, 146, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.E.A.; Saverymuttu, S.H.; Al-Sam, S.; Cook, M.G.; Maxwell, J.D. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin. Radiol. 1991, 43, 26–31. [Google Scholar] [CrossRef]
- Foster, K.J.; Dewbury, K.C.; Griffith, A.H.; Wright, R. The accuracy of ultrasound in the detection of fatty infiltration of the liver. Br. J. Radiol. 1979, 53, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Mottin, C.C.; Moretto, M.; Padoin, A.V.; Swarowsky, A.M.; Toneto, M.G.; Glock, L.; Repetto, G. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes. Surg. 2004, 14, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.K.; Johnson, L.A.; Germin, B.I.; Marcos, A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002, 8, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Ortiz Lopez, C.; Lomonaco, R.; Orsak, B.; Freckleton, M.; Chintapalli, K.; Hardies, J.; Lai, S.; Solano, F.; Tio, F.; et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 2015, 35, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.F.; Yan, H.M.; He, W.Y.; Li, X.M.; Li, C.L.; Yao, X.Z.; Li, R.K.; Zeng, M.S.; Gao, X. Standardized ultrasound hepatic/renal ratio and hepatic attenuation rate to quantify liver fat content: An improvement method. Obesity (Silver Spring) 2012, 20, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Ahmed, A. Obesity and non-alcoholic fatty liver disease: Disparate associations among Asian populations. World J. Hepatol. 2014, 6, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Schwenzer, N.F.; Springer, F.; Schraml, C.; Stefan, N.; Machann, J.; Schick, F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J. Hepatol. 2009, 51, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Bydder, G.M.; Chapman, R.W.G.; Harry, D.; Bassan, L.; Sherlock, S.; Kreel, L. Computed tomography attenuation values in fatty liver. J. Comput. Tomogr. 1981, 5, 33–35. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, P.N.; Kim, K.W.; Lee, S.W.; Yoon, S.E.; Park, S.W.; Ha, H.K.; Lee, M.-G.; Hwang, S.; Lee, S.-G.; et al. Macrovesicular hepatic steatosis in living liver donors: Use of CT for quantitative and qualitative assessment1. Radiology 2006, 239, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Ng, C.S.; Wu, T.T.; Ayers, G.D.; Curley, S.A.; Abdalla, E.K.; Vauthey, J.N.; Charnsangavej, C. Comparison of CT methods for determining the fat content of the liver. AJR Am. J. Roentgenol. 2007, 188, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Seppälä-Lindroos, A.; Vehkavaara, S.; Häkkinen, A.-M.; Goto, T.; Westerbacka, J.; Sovijärvi, A.; Halavaara, J.; Yki-Järvinen, H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J. Clin. Endocrinol. Metab. 2002, 87, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Westerbacka, J.; Bergholm, R.; Yki-Järvinen, H. Liver fat in the metabolic syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 3490–3497. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Kozakova, M.; Højlund, K.; Flyvbjerg, A.; Favuzzi, A.; Mitrakou, A.; Balkau, B. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 2009, 49, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Vehkavaara, S.; Yki-Järvinen, H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology 2008, 135, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Cusi, K.; Pettiti, M.; Hardies, J.; Miyazaki, Y.; Berria, R.; Buzzigoli, E.; Sironi, A.M.; Cersosimo, E.; Ferrannini, E.; et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007, 133, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Manley, S.E.; Stratton, I.M.; Clark, P.M.; Luzio, S.D. Comparison of 11 human insulin assays: Implications for clinical investigation and research. Clin. Chem. 2007, 53, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011, 53, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; You, W.; Zhang, H.; Peng, R.; Zhu, Q.; Yao, A.; Li, X.; Zhou, Y.; Wang, X.; Pu, L.; et al. PNPLA3 polymorphisms (rs738409) and non-alcoholic fatty liver disease risk and related phenotypes: A meta-analysis. J. Gastroenterol. Hepatol. 2015, 30, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Tao, A.; Zhang, S.; Deng, Y.; Chen, G. Association between patatin-like phospholipase domain containing 3 gene (PNPLA3) polymorphisms and nonalcoholic fatty liver disease: A HuGE review and meta-analysis. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Shen, J.-H.; Li, Y.-L.; Li, D.; Wang, N.-N.; Jing, L.; Huang, Y.-H. The rs738409 (I148M) variant of the PNPLA3 gene and cirrhosis: A meta-analysis. J. Lipid Res. 2015, 56, 167–175. [Google Scholar] [CrossRef]
- Trépo, E.; Nahon, P.; Bontempi, G.; Valenti, L.; Falleti, E.; Nischalke, H.D.; Hamza, S.; Corradini, S.G.; Burza, M.A.; Guyot, E.; et al. Association between the PNPLA3 (rs738409 C>G) variant and hepatocellular carcinoma: Evidence from a meta-analysis of individual participant data. Hepatology 2014, 59, 2170–2177. [Google Scholar] [CrossRef]
- He, S.; McPhaul, C.; Li, J.Z.; Garuti, R.; Kinch, L.; Grishin, N.V.; Hobbs, H.H. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J. Biol. Chem. 2010, 285, 6706–6715. [Google Scholar] [CrossRef]
- Huang, Y.; Cohen, J.C.; Hobbs, H.H. Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease. J. Biol. Chem. 2011, 286, 37085–37093. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Schoiswohl, G.; Chitraju, C.; Paar, M.; Cornaciu, I.; Rangrez, A.Y.; Wongsiriroj, N.; Nagy, H.M.; Ivanova, P.T.; Scott, S.A.; et al. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metab. 2012, 15, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Kantartzis, K.; Peter, A.; Machicao, F.; Machann, J.; Wagner, S.; Königsrainer, I.; Königsrainer, A.; Schick, F.; Fritsche, A.; Haring, H.-U.; et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes 2009, 58, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Johansson, L.E.; Johansson, L.M.; Roos, C.; Westerbacka, J.; Hamsten, A.; Bergholm, R.; Arkkila, P.; Arola, J.; Kiviluoto, T.; et al. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia 2009, 52, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; West, A.L.; Bhatia, L.; Hoile, S.P.; McCormick, K.G.; Burdge, G.C.; Lillycrop, K.A.; Clough, G.F.; Calder, P.C.; Byrne, C.D. Treating liver fat and serum triglyceride levels in NAFLD, effects of PNPLA3 and TM6SF2 genotypes: Results from the WELCOME trial. J. Hepatol. 2015, 63, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Wagenknecht, L.E.; Palmer, N.D.; Bowden, D.W.; Rotter, J.I.; Norris, J.M.; Ziegler, J.; Chen, Y.D.I.; Haffner, S.; Scherzinger, A.; Langefeld, C.D. Association of PNPLA3 with non-alcoholic fatty liver disease in a minority cohort: The insulin resistance atherosclerosis family study. Liver Int. 2011, 31, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Verrijken, A.; Beckers, S.; Francque, S.; Hilden, H.; Caron, S.; Zegers, D.; Ruppert, M.; Hubens, G.; Marck, E.; Michielsen, P.; et al. A gene variant of PNPLA3, but not of APOC3, is associated with histological parameters of NAFLD in an obese population. Obesity (Silver Spring) 2013, 21, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Gambino, R. PNPLA3 rs738409 and TM6SF2 rs58542926 gene variants affect renal disease and function in nonalcoholic fatty liver disease. Hepatology 2015, 62, 658–659. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Al-Serri, A.; Daly, A.K.; Galmozzi, E.; Rametta, R.; Dongiovanni, P.; Nobili, V.; Mozzi, E.; Roviaro, G.; Vanni, E.; et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2010, 51, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Del Ben, M.; Polimeni, L.; Brancorsini, M.; di Costanzo, A.; D’Erasmo, L.; Baratta, F.; Loffredo, L.; Pastori, D.; Pignatelli, P.; Violi, F.; et al. Non-alcoholic fatty liver disease, metabolic syndrome and patatin-like phospholipase domain-containing protein3 gene variants. Eur. J. Intern. Med. 2014, 25, 566–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.W.; Lin, H.Y.; Shin, S.J.; Yu, M.-L.; Lin, Z.-Y.; Dai, C.-Y.; Huang, J.-F.; Chen, S.-C.; Li, S.S.L.; Chuang, W.-L. The PNPLA3 I148M polymorphism is associated with insulin resistance and nonalcoholic fatty liver disease in a normoglycaemic population. Liver Int. 2011, 31, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cho, B.; Kwon, H.; Prilutsky, D.; Yun, J.M.; Choi, H.C.; Hwang, K.B.; Lee, I.H.; Kim, J.I.; Kong, S.W. I148M variant in PNPLA3 reduces central adiposity and metabolic disease risks while increasing nonalcoholic fatty liver disease. Liver Int. 2015, 35, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Chang, P.-F.; Hu, F.-C.; Yang, W.-S.; Chang, M.-H.; Ni, Y.-H. A Common variant in the PNPLA3 gene is a risk factor for Non-alcoholic fatty liver disease in obese Taiwanese children. J. Pediatr. 2011, 158, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Sentinelli, F.; Cambuli, V.M.; Incani, M.; Congiu, T.; Matta, V.; Pilia, S.; Huang-Doran, I.; Cossu, E.; Loche, S.; et al. The 148M allele of the PNPLA3 gene is associated with indices of liver damage early in life. J. Hepatol. 2010, 53, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Alisi, A.; Galmozzi, E.; Bartuli, A.; del Menico, B.; Alterio, A.; Dongiovanni, P.; Fargion, S.; Nobili, V. I148M patatin-like phospholipase domain-containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology 2010, 52, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Pirola, C.J.; Sookoian, S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: A meta-analysis. Hepatology 2015, 62, 1742–1756. [Google Scholar] [CrossRef] [PubMed]
- Smagris, E.; Gilyard, S.; BasuRay, S.; Cohen, J.C.; Hobbs, H.H. Inactivation of TM6SF2, a gene defective in fatty liver disease, impairs lipidation but not secretion of very low density lipoproteins. J. Biol. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mahdessian, H.; Taxiarchis, A.; Popov, S.; Silveira, A.; Franco-Cereceda, A.; Hamsten, A.; Eriksson, P.; van’t Hooft, F. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proc. Natl. Acad. Sci. USA 2014, 111, 8913–8918. [Google Scholar] [CrossRef] [PubMed]
- Holmen, O.L.; Zhang, H.; Fan, Y.; Hovelson, D.H.; Schmidt, E.M.; Zhou, W.; Guo, Y.; Zhang, J.; Langhammer, A.; Løchen, M.-L.; et al. Systematic evaluation of coding variation identifies a candidate causal variant in TM6SF2 influencing total cholesterol and myocardial infarction risk. Nat. Genet. 2014, 46, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Llauradó, G.; Orešič, M.; Hyötyläinen, T.; Orho-Melander, M.; Yki-Järvinen, H. Circulating triacylglycerol signatures and insulin sensitivity in NAFLD associated with the E167K variant in TM6SF2. J. Hepatol. 2015, 62, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Mangia, A.; Berg, T.; Chan, H.L.-Y.; Irving, W.L.; Dore, G.J.; Abate, M.L.; Bugianesi, E.; Adams, L.A.; Najim, M.A.M.; et al. Diverse impacts of the rs58542926 E167K variant in TM6SF2 on viral and metabolic liver disease phenotypes. Hepatology 2016. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, M.; Caprio, S.; Feldstein, A.E.; D’Adamo, E.; Shaw, M.M.; Pierpont, B.; Savoye, M.; Zhao, H.; Bale, A.E.; Santoro, N. Role of TM6SF2 rs58542926 in the pathogenesis of nonalcoholic pediatric fatty liver disease: A multiethnic study. Hepatology 2016, 63, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Grandone, A.; Cozzolino, D.; Marzuillo, P.; Cirillo, G.; di Sessa, A.; Ruggiero, L.; di Palma, M.R.; Perrone, L.; Miraglia del Giudice, E. TM6SF2 Glu167Lys polymorphism is associated with low levels of LDL-cholesterol and increased liver injury in obese children. Pediatr. Obes. 2016, 11, 115–119. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016, 9, 65–90. [Google Scholar]

| Study | Year | N | Subjects | Normal Value |
|---|---|---|---|---|
| Biochemical | ||||
| Laurell S [21] | 1971 | 3 | Healthy subjects | 2.0 g/100 g of dry tissue weight |
| Donhoffer H [15] | 1974 | 107 | Unselected cadavers | 5.5 g/100 g of wet tissue weight |
| Histology | ||||
| Kleiner DE [17] | 2005 | 576 + 162 | Adults and children | Macroscopic fat in <5% of hepatocytes |
| Brunt EM [3] | 2011 | 976 | Adults | Macroscopic fat in <5% of hepatocytes |
| Bedossa P [19] | 2012 | 679 | Morbidly obese adults | Macroscopic fat in <5% of hepatocytes |
| CT | ||||
| Piekarski J [22] | 1980 | 100 | Healthy subjects | 50–57 HU or 8–10 HU higher than spleen |
| 1H-MRS | ||||
| Szczepaniak LS [23] | 2005 | 345 | Population-based, healthy subjects | <5.56% |
| Petersen KF [24] | 2006 | 170 | Healthy subjects | <3.0% |
| MRI-PDFF | ||||
| Fishbein MH [25] | 1998 | 28 | Healthy subjects | <9.0% |
| US | ||||
| Joseph AE [26] | 1978 | 60 | Adults referred to gastroenterologist | Absense of echogenicity or brightness of the liver |
| Saveymuttu SH [27] | 1985 | 490 | Adults referred to gastroenterologist | Absense of echogenicity or brightness of the liver |
| Cohort | N | BMI (kg/m2) | Liver Fat | Insulin Sensitivity (HOMA-IR) | S-Triglycerides (mmol/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I148II | I148IM | I148MM | I148II | I148IM | I148MM | I148II | I148IM | I148MM | I148II | I148IM | I148MM | ||
| Multiethnic 1 [12] | 2111 | 30.4 | 31.1 | 30.0 | 3.7% a | 4.6% a | 7.7% ***,a | 3.3 | 3.5 | 3.3 | 1.32 | 1.35 | 1.41 |
| 31.6 | 32.0 | 32.2 | 3.1% | 4.8% | 4.8% *** | 3.3 | 3.3 | 4.4 | 0.97 | 0.97 | 1.02 | ||
| 29.2 | 28.8 | 28.8 | 3.5% | 3.7% | 3.5% *** | 2.3 | 2.4 | 2.0 | 1.25 | 1.21 | 0.90 | ||
| Germany [79] | 330 | 29.9 | 29.1 | 28.7 | 5.4% a | 6.0% a | 7.2% ***,a | 12.6 v,z | 12.9 v,z | 12.9 v,z | NA | NA | NA |
| Finnish [80] | 291 | 30.5 | 30.0 | 32.2 | 9.0% a | 10.4% *,a | 14.1% **,a | 72 y,z | 70 y,z | 74 y,z | 1.82 | 1.60 | 1.52 |
| British [81] | 98 | 34.6 | 33.2 | 31.7 | 26.7% a | 28.8% a | 33.5% a | 2.4 | 3.1 | 2.6 | 1.60 | 1.70 | 1.40 |
| Multiethnic 2 [82] | 1214 | NA × | NA × | NA × | 57 b | 55 b | 46 ***,b | NA × | NA× | NA× | NA × | NA × | NA × |
| 55 | 51 | 47 *** | |||||||||||
| Dutch [83] | 470 | 37.7 | 37.6 | 37.6 | 66% c | 78% c | 100% ***,c | 2.7 | 2.8 | 2.9 | 1.42 | 1.47 | 1.46 |
| Italian [84] | 61 | 25.7 | 25.9 | 16% d | 32% *,d | 3.4 | 4.7 | 1.13 | 1.15 | ||||
| Italian [85] | 253 | 30.7 | 30.7 | 29.8 | 44% c | 48% c | 63% **,c | 3.9 | 4 | 5.2 | 1.64 | 1.85 | 1.79 |
| Italian [86] | 211 | 32.1 | 30.4 | 31.7 | 4 e | 4 e | 4 e | 3.5 | 3.5 | 2.8 | 1.77 | 1.59 | 1.26 ** |
| Taiwanese [87] | 879 | 23.3 | 23.6 | 23.6 | 13% f | 19% f | 23% *,f | 1.4 | 1.5 | 1.5 | 1.11 | 1.16 | 1.38 * |
| South Korean [88] | 1363 | 24.7 | 24.4 | 23.9 ** | 38% f | 45% f | 54% *,f | 2.3 | 2.1 | 1.6 ** | 1.54 | 1.38 | 1.31 ** |
| Taiwanese, pediatric [89] | 520 | 26.3 | 26.2 | 25.9 | 21% f | 13% f | 30% **,f | 2.4 | 2.5 | 1.7 | 1.11 | 1.03 | 0.94 |
| Italian, pediatric [90] | 475 | NA | NA | NA | 13% f | 19% f | 41% *,f | 3.3 | 3.0 | 3.0 | 0.56 | 0.56 | 0.53 |
| Italian, pediatric [91] | 149 | 95.2 | 95.0 | 94.1 | 70% g | 7% g | 4% ***,g | 2.5 | 2.7 | 2.4 | 1.28 | 1.19 | 1.39 |
| 30% | 78% | 4% | |||||||||||
| 0% | 15% | 92% | |||||||||||
| Cohort | N | BMI (kg/m2) | Liver Fat | Insulin Sensitivity (HOMA-IR) | S-Triglycerides (mmol/L) | ||||
|---|---|---|---|---|---|---|---|---|---|
| EE | EK + KK | EE | EK + KK | EE | EK + KK | EE | EK + KK | ||
| Multiethnic 1 [92] | 4587 | 29.6 | 28.5/31.8 | 3.5% a | 4.4%/15.7% ***,a | 3.0 | 2.9/4.6 | 1.39 | 1.33/1.47 * |
| Finns [97] | 300 | 33.7 | 32.5 | 6.8% a | 11.2% *,a | 3.0 | 2.9 | 1.40 | 1.50 |
| British [81] | 98 | 32.6 | 35.4 | 28.5% a | 29.0% a | 2.7 | 4.0 | 1.60 | 1.50 * |
| Argentineans [13] | 361 | 29.8 | 30.2 | NA | NA | 3.1 | 3.0 | 1.87 | 1.31 |
| Multiethnic 2 [98] | 502 | 32.2 | 31.2/30.8 | S0: 3% b | S0: 0%/0% b | 3.5 | 2.8/2.8 | 1.70 | 1.36/1.08 ** |
| S1: 50% | S1: 35%/45% | ||||||||
| S2: 27% | S2: 40%/20% | ||||||||
| S3: 20% | S3: 25%/35% * | ||||||||
| Multiethnic 1, pediatric [99] | 957 ^ | 33.0 | 32.6 | 6.7% c ^ | 11.1% **,c,^ | 1.9 x | 2.0 x | 1.20 | 1.21 |
| Italian, pediatric [100] | 1010 | 2.9 | 2.9 | 47% d | 89% **,d | 5.6 | 4.6 | 1.12 | 1.02 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petäjä, E.M.; Yki-Järvinen, H. Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD—A Systematic Review. Int. J. Mol. Sci. 2016, 17, 633. https://doi.org/10.3390/ijms17050633
Petäjä EM, Yki-Järvinen H. Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD—A Systematic Review. International Journal of Molecular Sciences. 2016; 17(5):633. https://doi.org/10.3390/ijms17050633
Chicago/Turabian StylePetäjä, Elina M., and Hannele Yki-Järvinen. 2016. "Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD—A Systematic Review" International Journal of Molecular Sciences 17, no. 5: 633. https://doi.org/10.3390/ijms17050633
APA StylePetäjä, E. M., & Yki-Järvinen, H. (2016). Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD—A Systematic Review. International Journal of Molecular Sciences, 17(5), 633. https://doi.org/10.3390/ijms17050633