Degree of Conversion and BisGMA, TEGDMA, UDMA Elution from Flowable Bulk Fill Composites
Abstract
:1. Introduction
2. Results
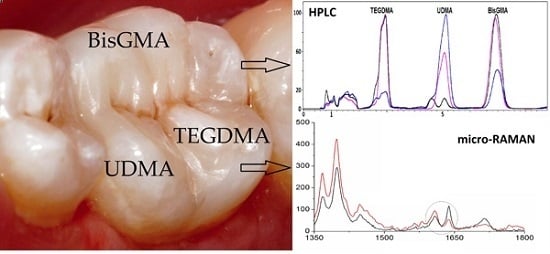
2.1. Degree of Conversion-Micro-Raman Spectroscopy
2.2. Monomer Elution: HPLC
3. Discussion
4. Materials and Methods
4.1. Preparation of the Composite Resin Specimens
4.2. Micro-Raman Spectroscopy Measurement
4.3. RP-HPLC Measurements
4.4. Validation of the Monomer Determination the Limit of Detection and the Limit of Quantification
4.5. Statistical Analysis
5. Conclusions
- (1)
- Among the investigated low viscosity bulk fill and conventional flowable RBCs, SDR showed the highest DC value at the top and bottom surface of the samples.
- (2)
- The DC values of the 4 mm-thick bulk-fill composites SDR, FBF, XB were significantly higher than that of the 4 mm-thick conventional composite (negative control) studied; meanwhile, only SDR bulk-fill resulted in a higher DC value compared to that of the 2 mm-thick conventional flowable RBC (positive control).
- (3)
- Although the recommended exposure time by the manufacturers for the universal shade FBF and XB is 10 s (with a 1000-mW/cm2 curing unit), extended (20 s) curing time significantly increased the DC% value.
- (4)
- The amount of released BisGMA and TEGDMA monomers from the bulk-fill composite materials was generally lower than from the conventional composite.
- (5)
- Among bulk fills, in spite of the highest DC%, SDR showed the highest rate of TEGDMA elution; meanwhile, the highest amount of UDMA was eluted from FBF.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DC | degree of conversion |
| HPLC | high performance liquid chromatography |
| RP-HPLC | reverse-phase high-performance liquid chromatography |
| SDR | SureFil SDR Flow |
| XB | X-tra Base |
| FBF | Filtek Bulk Fill |
| FUF | Filtek Ultimate Flow |
| FUF_2mm_20s | Filtek Ultimate Flow in a 2-mm layer thickness cured for 20 s |
| FUF_4mm_20s | Filtek Ultimate Flow in a 4-mm layer thickness cured for 20 s |
| FBF_4mm_10s | 4 mm-thick Filtek Bulk Fill light cured for 10 s |
| FBF_4mm_20s | 4 mm-thick Filtek Bulk Fill light cured for 20 s |
| XB_4mm_10s | 4 mm-thick X-tra Base light cured for 10 s |
| XB_4mm_20s | 4 mm-thick X-tra Base light cured for 20 s |
| SDR_4mm_20s | SureFil SDR Flow in a 4-mm layer thickness cured for 20 s |
| UDMA | urethane dimethacrylate |
| BisGMA | bisphenol A diglycidyl ether dimethacrylate |
| TEGDMA | triethylene glycol dimethacrylate |
| BisEMA | bisphenol A polyethylene glycol diether dimethacrylate |
| EBPADMA | ethoxylated bisphenol A dimethacrylate |
| RBC | resin-based composite |
| SD | standard deviation |
| QTH | quartz tungsten halogen |
| LCU | light curing unit |
| CQ | camphorquinone |
| CAN | acetonitrile |
| LED | light emitting diode |
| LOD | limit of detection |
| LOQ | limit of quantification |
References
- Da Rosa Rodolpho, P.A.; Donassollo, T.A.; Cenci, M.S.; Loguércio, A.D.; Moraes, R.R.; Bronkhorst, E.M.; Opdam, N.J.M.; Demarco, F.F. 22-year clinical evaluation of the performance of two posterior composites with different filler characteristics. Dent. Mater. 2011, 27, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Lempel, E.; Tóth, Á.; Fábián, T.; Krajczár, K.; Szalma, J. Retrospective evaluation of posterior direct composite restorations: 10-Year findings. Dent. Mater. 2015, 31, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Opdam, N.J.M.; Bronkhorst, E.M.; Loomans, B.A.; Hujsmans, M.C. 12-year survival of composite vs. amalgam restorations. J. Dent. Res. 2010, 89, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, U.; van Dijken, J.W.V.; Halken, J.; Hallosten, A.L.; Höigaard, R. Longevity of posterior resin composite restorations in permanent teeth in Public Dental Health Service: A prospective 8 years follow up. J. Dent. 2013, 41, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Elution of leachable components from composites. J. Oral Rehabil. 1994, 21, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Greener, E.H. The effect of resin formulation on the degree of conversion and mechanical properties of dental restorative resins. J. Biomed. Mater. Res. 1986, 20, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Lempel, E.; Czibulya, Z.; Kunsági-Máté, S.; Szalma, J.; Sümegi, B.; Böddi, K. Quantification of conversion degree and monomer elution from dental composite using HPLC and micro-raman spectroscopy. Chromatographia 2014, 77, 1137–1144. [Google Scholar] [CrossRef]
- Poggio, C.; Lombardini, M.; Gaviati, S.; Chiesa, M. Evaluation of Vickers hardness and depth of cure of six composite resins photo-activated with different polymerization modes. J. Conserv. Dent. 2012, 15, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Silikas, N.; Eliades, G.; Watts, D.C. Light intensity effects on resin-composite degree of conversion and shrinkage strain. Dent. Mater. 2000, 16, 292–296. [Google Scholar] [CrossRef]
- Turssi, C.P.; Ferracane, J.L.; Vogel, K. Filler features and their effects on wear and degree of conversion of particulate dental resin composites. Biomaterials 2005, 26, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Jelen, E.; Hickel, R. Is the soft-start polymerization concept still relevant for modern curing units? Clin. Oral Investig. 2011, 15, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Alomari, Q.D.; Reinhardt, J.W.; Boyer, D.B. Effect of liners on cusp deflection and gap formation in composite restorations. Oper. Dent. 2001, 26, 406–411. [Google Scholar] [PubMed]
- Park, J.; Chang, J.; Ferracane, J.; Lee, I.B. How should composite be layered to reduce shrinkage stress: Incremental or bulk filling. Dent. Mater. 2008, 24, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Obici, A.C.; Sinhoreti, M.A.C.; Frollini, E.; Correr-Sobrinho, L.; Fernando de Goes, M.; Henriques, G.E.P. Monomer conversion at different dental composite depths using six light-curing methods. Polym. Test. 2006, 25, 282–288. [Google Scholar] [CrossRef]
- Watts, D.C.; Cash, A.J. Analysis of optical transmission by 400–500 nm visible light into aesthetic dental biomaterials. J. Dent. 1994, 22, 112–117. [Google Scholar] [CrossRef]
- Flury, S.; Hayoz, S.; Peutzfeldt, A.; Hüsler, J.; Lussi, A. Depth of cure of resin composites: Is the ISO 4049 method suitable for bulk fill materials? Dent. Mater. 2012, 28, 521–528. [Google Scholar] [CrossRef] [PubMed]
- El-Safty, S.; Silikas, N.; Watts, D.C. Creep deformation of restorative resin-composites intended for bulk-fill placement. Dent. Mater. 2012, 28, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Bucuta, S.; Ilie, N. Light transmittance and micro-mechanical properties of bulk fill vs. conventional resin based composites. Clin. Oral Investig. 2014, 18, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Finan, L.; Palin, W.M.; Moskwa, N.; McGinley, E.L.; Fleming, G.J.P. The influence of irradiation potential on the degree of conversion and mechanical properties of two bulk-fill flowable RBC base materials. Dent. Mater. 2013, 29, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Van Ende, A.; De Munch, J.; Van Landuyt, K.L.; Poitevin, A.; Peumans, M.; Van Meerbeek, B. Bulk-filling of high C-factor posterior cavities, effect on adhesion to cavity bottom dentin. Dent. Mater. 2013, 29, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Campodonico, C.E.; Tantbirojn, D.; Olin, P.S.; Versluis, A. Cuspal deflection and depth of cure in resin-based composite restorations filled by using bulk; incremental and transtooth illumination techniques. J. Am. Dent. Assoc. 2011, 142, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Roggendorf, M.J.; Kramer, N.; Appelt, A.; Naumann, M.; Frankenberger, R. Marginal quality of flowable 4-mm base vs conventionally layered resin composite. J. Dent. 2011, 39, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Hickel, R. Investigations on a methacrylate-based flowable composite based on the SDR™ technology. Dent. Mater. 2011, 27, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, A.; Hogg, C.H.; Dowling, A.H.; Grufferty, B.F.; Benetti, A.R.; Fleming, G.J.P. Cuspal deflection and microleakage in premolar teeth restored with bulk-fill flowable resin-based composite base materials. J. Dent. 2012, 40, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Czasch, P.; Ilie, N. In vitro comparison of mechanical properties and degree of cure of bulk fill composites. Clin. Oral Investig. 2013, 17, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Van Dijken, J.W.V.; Pallesen, U. A randomized controlled three year evaluation of “bulk-filled” posterior resin restorations based on stress decreased resin technology. Dent. Mater. 2014, 30, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M. In vitro and in vivo studies on the toxicity of dental resin components, a review. Clin. Oral Investig. 2008, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Mourelatos, D.; Tsiftsoglou, A.S.; Giassin, N.P.; Mioglou, E.; Garefis, P. Genotoxic and cytotoxic effects of different types of dental cement on normal cultured human lymphocytes. Mutat. Res. 2009, 672, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.S.; Almeida, G.S.; Poskus, L.T.; Guimarães, J.G. Relationship between the degree of conversion; solubility and salivary sorption of a hybrid and nanofilled resin composite: Influence of the light activation mode. Appl. Oral Sci. 2008, 16, 161–166. [Google Scholar] [CrossRef]
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent advances and developments in composite dental restorative materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Leprince, J.G.; Palin, W.M.; Hadis, M.A.; Devaux, J.; Leloup, G. Progress in dimethacrylate-based dental composite technology and curing efficiency. Dent. Mater. 2013, 29, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Alrahlah, A.; Silikas, N.; Watts, D.C. Post-cure depth of cure of bulk fill dental resin-composites. Dent. Mater. 2014, 30, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Shortall, A.C. How light source and product shade influence cure depth for a contemporary composite. J. Oral Rehabil. 2005, 32, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K. Influence of filler on difference between the transmitted and reflected colors of experimental resin composites. Dent. Mater. 2008, 24, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, R.H.; Erickson, R.L.; Davidson, C.L. The effect of filler and silane content on conversion of resin-based composite. Dent. Mater. 2003, 19, 327–333. [Google Scholar] [CrossRef]
- Nomoto, R.; Hirasawa, T. Residual monomer and pendant methacryloyl group in light-cured composite resins. Dent. Mater. J. 1992, 11, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Silikas, N.; Satterthwaite, J.D. Degree of conversion of bulk-fill compared to conventional resin-composites at two time intervals. Dent. Mater. 2013, 29, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Tarle, Z.; Attin, T.; Marovic, D.; Andermatt, L.; Ristic, M.; Tauböck, T.T. Influence of irradiation time on subsurface degree of conversion and microhardness of high-viscosity bulk-fill resin composites. Clin. Oral Investig. 2015, 19, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pongprueksa, P.; van Meerbeek, B.; de Munk, J. Curing profile of bulk-fill resin-based composites. J. Dent. 2015, 43, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.; Söderholm, K.J.M. How light irradiance and curing time affect monomer conversion in light-cured resin composites. Eur. J. Oral Sci. 2003, 111, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Zorzin, J.; Maier, E.; Harre, S.; Fey, T.; Belli, R.; Lohbauer, U.; Petschelt, A.; Taschner, M. Bulk-fill resin composites: Polymerization properties and extended light curing. Dent. Mater. 2015, 31, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Baroudi, K.; Saleh, A.M.; Silikas, N.; Watts, D.C. Shrinkage behaviour of flowable resin-composites related to conversion and filler-fraction. J. Dent. 2007, 35, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.D.; Karabela, M.M. Effect of the amount of 3-methacryloxypropyltrimethoxysilane coupling agent on physical properties of dental resin nanocomposites. Dent. Mater. 2009, 25, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Asmussen, E.; Peutzfeldt, A. Influence of UEDMA, BisGMA and TEGDMA on selected mechanical properties of experimental resin composites. Dent. Mater. 1998, 14, 51–56. [Google Scholar] [CrossRef]
- Khatri, C.A.; Stansbury, J.W.; Schultheisz, C.R.; Antonucci, J.M. Synthesis characterization and evaluation of urethane derivates of Bis-GMA. Dent. Mater. 2003, 19, 584–588. [Google Scholar] [CrossRef]
- Uzunova, Y.; Lukanov, L.; Filipov, I.; Vladimirov, S. High-performance liquid chromatographic determination of unreacted monomers and other residues contained in dental composites. J. Biomech. Biophys. Methods 2008, 70, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Caughmann, W.F.; Caughmann, G.B.; Shiflett, R.; Rueggeberg, F.; Schuster, G. Correlation of cytotoxicity, filler loading and curing time of dental composites. Biomaterials 1991, 12, 737–740. [Google Scholar] [CrossRef]
- Tanaka, K.; Taira, M.; Shintani, H.; Wakasa, K.; Yamaki, M. Residual monomers (TEGDMA and Bis-GMA) of a set visible-light-cured dental composite resin when immersed in water. J. Oral Rehabil. 1991, 18, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Cebe, M.A.; Cebe, F.; Cengiz, M.F.; Cetin, A.R.; Arpag, O.F.; Ozturk, B. Elution of monomer from different bulk fill dental composite resin. Dent. Mater. 2015, 31, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Łagocka, R.; Jakubowska, K.; Chlubek, D.; Buczkowska-Radlińska, J. Elution study of unreacted TEGDMA from bulk-fill composite (SDR™ Dentsply) using HPLC. Adv. Med. Sci. 2015, 60, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Ortengren, U.; Wellendorf, H.; Karlsson, S.; Ruyter, IE. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J. Oral Rehabil. 2001, 28, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Braden, M.; Clarke, R.L. Water absorption characteristics of dental microfine composite filling materials. I. Proprietary materials. Biomaterials 1984, 5, 369–372. [Google Scholar] [CrossRef]
- Braden, M.; Davy, K.W.M. Water absorption characteristics of some unfilled resins. Biomaterials 1986, 7, 474–475. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Karabela, M.M.; Sideridou, I.D. Effect of the structure of silane coupling agent on sorption characteristics of solvents by dental resin-nanocomposites. Dent. Mater. 2008, 24, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Salim, N.A.; Sung, R.; Satterthwaite, J.D.; Silikas, N. Qualitative and quantitative characterization of monomers of uncured bulk-fill and conventional resin-composites using liquid chromatography/mass spectrometry. Dent. Mater. 2015, 31, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry, Reactions, Mechanisms and Structure, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Durner, J.; Schrickel, K.; Watts, D.C.; Ilie, N. Determination of homologous distributions of BisEMAdimethacrylates in bulk-fill resin-composites by GC-MS. Dent. Mater. 2015, 31, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Santini, A.; Miletic, V.; Koutsaki, D. Degree of conversion of three fissure sealants cured by different light curing units using micro-Raman spectroscopy. J. Dent. Sci. 2012, 7, 26–32. [Google Scholar] [CrossRef]
- Ogliari, F.A.; Ely, C.; Zanchi, C.H.; Fortes, C.B.B.; Samuel, S.M.W.; Demarco, F.F.; Petzhold, C.L.; Piva, E. Influence of chain extender length of aromatic dimethacrylates on polymer network development. Dent. Mater. 2008, 20, 165–171. [Google Scholar] [CrossRef] [PubMed]



| Group | Material | Code | Manufacturer | Shade | Organic Matrix | Filler | Filler Loading | LOT Number |
|---|---|---|---|---|---|---|---|---|
| Bulk-fill composite | SureFil SDR Flow | SDR | Dentsply Caulk, Milford, DE, USA | U | Modified UDMA, EBPADMA, TEGDMA | Ba-Al-F-B silicate glass, Sr-Al-F silicate glass | 68 wt % | 1202174 |
| x-tra base | XB | Voco, Cuxhaven, Germany | U | UDMA, BisEMA | no information | 75 wt % | 1305261 | |
| Filtek Bulk Fill | FBF | 3M ESPE, St Paul, MN, USA | U | BisGMA, UDMA, BisEMA(6), TEGDMA, substituated dimethacrylate, Procrylat resin | silane treated zirconia/silica, ytterbium trifluoride | 64.5 wt % | N414680 | |
| Conventional flowable composite | Filtek Ultimate Flow | FUF | 3M ESPE, St Paul, MN, USA | A2 | BisGMA, TEGDMA, substituated dimethacrylate, Procrylat resin | silane treated zirconia/silica, ytterbium trifluoride | 65 wt % | N652740 |
| Abbreviation | Material | Layer Thickness (mm) | Exposure Time (s) |
|---|---|---|---|
| FUF_2mm_20s | Filtek Ultimate Flow | 2 | 20 |
| FUF_4mm_20s | Filtek Ultimate Flow | 4 | 20 |
| FBF_4mm_10s | Filtek Bulk Fill | 4 | 10 |
| FBF_4mm_20s | Filtek Bulk Fill | 4 | 20 |
| XB_4mm_10s | X-tra Base | 4 | 10 |
| XB_4mm_20s | X-tra Base | 4 | 20 |
| SDR_4mm_20s | SureFil SDR Flow | 4 | 20 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lempel, E.; Czibulya, Z.; Kovács, B.; Szalma, J.; Tóth, Á.; Kunsági-Máté, S.; Varga, Z.; Böddi, K. Degree of Conversion and BisGMA, TEGDMA, UDMA Elution from Flowable Bulk Fill Composites. Int. J. Mol. Sci. 2016, 17, 732. https://doi.org/10.3390/ijms17050732
Lempel E, Czibulya Z, Kovács B, Szalma J, Tóth Á, Kunsági-Máté S, Varga Z, Böddi K. Degree of Conversion and BisGMA, TEGDMA, UDMA Elution from Flowable Bulk Fill Composites. International Journal of Molecular Sciences. 2016; 17(5):732. https://doi.org/10.3390/ijms17050732
Chicago/Turabian StyleLempel, Edina, Zsuzsanna Czibulya, Bálint Kovács, József Szalma, Ákos Tóth, Sándor Kunsági-Máté, Zoltán Varga, and Katalin Böddi. 2016. "Degree of Conversion and BisGMA, TEGDMA, UDMA Elution from Flowable Bulk Fill Composites" International Journal of Molecular Sciences 17, no. 5: 732. https://doi.org/10.3390/ijms17050732
APA StyleLempel, E., Czibulya, Z., Kovács, B., Szalma, J., Tóth, Á., Kunsági-Máté, S., Varga, Z., & Böddi, K. (2016). Degree of Conversion and BisGMA, TEGDMA, UDMA Elution from Flowable Bulk Fill Composites. International Journal of Molecular Sciences, 17(5), 732. https://doi.org/10.3390/ijms17050732