How Diet Intervention via Modulation of DNA Damage Response through MicroRNAs May Have an Effect on Cancer Prevention and Aging, an in Silico Study
Abstract
:1. Introduction
2. Results
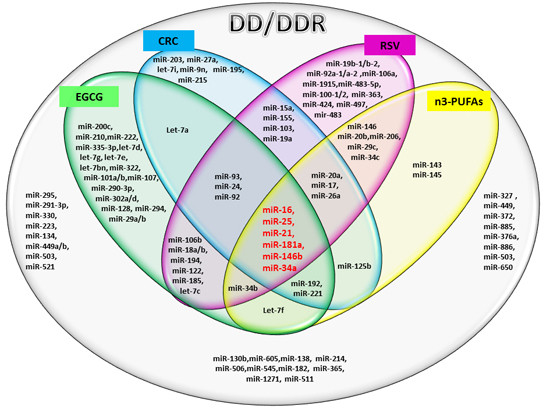
2.1. miRs Involved in DD/DDR and Bioactive Compounds Modulated
2.2. In Silico Analysis of Pathways Shared by Different miRs Involved in DD/DDR and Modulated by Compounds
3. Discussion
4. Materials and Methods
4.1. Search Strategy and Selection Criteria
4.2. In Silico Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EGCG | Epi-gallocatechin-3-gallate |
| RSV | Resveratrol |
| CRC | Curcumin |
| n3-PUFAs | n3-polyunsaturated fatty acids |
| miR | micro-RNA |
| DD | DNA damage |
| DDR | DNA damage response |
References
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Herbig, U.; Longhese, M.P.; Fumagalli, M.; d’Adda di Fagagna, F. Irreparable telomeric DNA damage and persistent DDR signalling as a shared causative mechanism of cellular senescence and ageing. Curr. Opin. Genet. Dev. 2014, 26, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, B.M.; Cortez, D. DNA damage response: Three levels of DNA repair regulation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012724. [Google Scholar] [CrossRef] [PubMed]
- Malaquin, N.; Carrier-Leclerc, A.; Dessureault, M.; Rodier, F. DDR-mediated crosstalk between DNA-damaged cells and their microenvironment. Front. Genet. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gatti, R.A. MicroRNAs: New players in the DNA damage response. J. Mol. Cell Biol. 2011, 3, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.A.; Shaposhnikov, M.V.; Plyusnina, E.N.; Zhavoronkov, A.; Budovsky, A.; Yanai, H.; Fraifeld, V.E. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res. Rev. 2013, 12, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, F.; Albertini, M.C.; Orciani, M.; Ceka, A.; Cricca, M.; Procopio, A.D.; Bonafe, M. DNA damage response (DDR) and senescence: Shuttled inflamma-miRNAs on the stage of inflamm-aging. Oncotarget 2015, 6, 35509–35521. [Google Scholar] [PubMed]
- Zhang, C.; Peng, G. Non-coding RNAs: An emerging player in DNA damage response. Mutat. Res. Rev. Mutat. Res. 2015, 763, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Chao, T.; Tu, K.; Zhang, Y.; Xie, L.; Gong, Y.; Yuan, J.; Qiang, B.; Peng, X. Improving the prediction of human microRNA target genes by using ensemble algorithm. FEBS Lett. 2007, 581, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Nie, Q.; Zhang, X. MicroRNAs involved in skeletal muscle differentiation. J. Genet. Genom. 2013, 40, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, Y.; Hao, Y.; Juan, L.; Teng, M.; Zhang, X.; Li, M.; Wang, G.; Liu, Y. MiR2 Disease: A manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009, 37, D98–D104. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.E. Targeting of mrnas by multiple miRNAs: The next step. Oncogene 2010, 29, 2161–2164. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.S.; Ferguson, L.R. The interaction between epigenetics, nutrition and the development of cancer. Nutrients 2015, 7, 922–947. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Friso, S. Epigenetics: A new bridge between nutrition and health. Adv. Nutr. 2010, 1, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Chedraui, P.; Perez-Lopez, F.R. Nutrition and health during mid-life: Searching for solutions and meeting challenges for the aging population. Climacteric 2013, 16, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Ghorbani, A. Cancer therapy with phytochemicals: Evidence from clinical studies. Avicenna J. Phytomed. 2015, 5, 84–97. [Google Scholar] [PubMed]
- Farago, N.; Feher, L.Z.; Kitajka, K.; Das, U.N.; Puskas, L.G. MicroRNA profile of polyunsaturated fatty acid treated glioma cells reveal apoptosis-specific expression changes. Lipids Health Dis. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Leyva, D.; Dupasquier, C.M.; McCullough, R.; Pierce, G.N. The cardiovascular effects of flaxseed and its omega-3 fatty acid, α-linolenic acid. Can. J. Cardiol. 2010, 26, 489–496. [Google Scholar] [CrossRef]
- Carotenuto, F.; Minieri, M.; Monego, G.; Fiaccavento, R.; Bertoni, A.; Sinigaglia, F.; Vecchini, A.; Carosella, L.; di Nardo, P. A diet supplemented with Ala-rich flaxseed prevents cardiomyocyte apoptosis by regulating caveolin-3 expression. Cardiovasc. Res. 2013, 100, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, F.; Costa, A.; Albertini, M.C.; Rocchi, M.B.L.; Rudov, A.; Coletti, D.; Minieri, M.; di Nardo, P.; Teodori, L. Dietary flaxseed mitigates impaired skeletal muscle regeneration: In vivo, in vitro and in silico studies. Int. J. Med. Sci. 2016, 13, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Ruijters, E.J.; Haenen, G.R.; Willemsen, M.; Weseler, A.R.; Bast, A. Food-derived bioactives can protect the anti-inflammatory activity of cortisol with antioxidant-dependent and -independent mechanisms. Int. J. Mol. Sci. 2016, 17, 239. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Soule, B.P.; Simone, B.A.; Zaorsky, N.G.; Jin, L.; Simone, N.L. MicroRNA expression altered by diet: Can food be medicinal? Ageing Res. Rev. 2014, 17, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.; Bontempo, P.; Nebbioso, A.; Altucci, L. Natural compounds in epigenetics: A current view. Food Chem. Toxicol. 2014, 73, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-mirpath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Mathur, R.; Hu, X.; Zhang, X.; Lu, X. miRNA response to DNA damage. Trends Biochem. Sci. 2011, 36, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Vrijens, K.; Bollati, V.; Nawrot, T.S. MicroRNAs as potential signatures of environmental exposure or effect: A systematic review. Environ. Health Perspect. 2015, 123, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.P.; Kwok, T.T. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J. Nutr. Biochem. 2010, 21, 140–146. [Google Scholar] [CrossRef] [PubMed]
- An, I.S.; An, S.; Park, S.; Lee, S.N.; Bae, S. Involvement of MicroRNAs in epigallocatechin gallate-mediated UVB protection in human dermal fibroblasts. Oncol. Rep. 2013, 29, 253–259. [Google Scholar] [PubMed]
- Toden, S.; Tran, H.M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bian, S.; Yang, C.S. Green tea polyphenol egcg suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis 2011, 32, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.H.; Wang, X.; Feng, Q. EGCG enhances the efficacy of cisplatin by downregulating HSA-miR-98-5p in NSCLC A549 cells. Nutr. Cancer 2014, 66, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wang, W. Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1. Tumour Biol. 2016, 37, 4373–4382. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tao, C.; He, A.; He, X. Overexpression of miR-126 sensitizes osteosarcoma cells to apoptosis induced by epigallocatechin-3-gallate. World J. Surg. Oncol. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Mekky, R.Y.; El-Ekiaby, N.M.; Hamza, M.T.; Elemam, N.M.; El-Sayed, M.; Esmat, G.; Abdelaziz, A.I. miR-194 is a hepatocyte gate keeper hindering HCV entry through targeting CD81 receptor. J. Infect. 2015, 70, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Khandkar, M.; Banik, N.L.; Ray, S.K. Alterations in expression of specific MicroRNAs by combination of 4-HPR and EGCG inhibited growth of human malignant neuroblastoma cells. Brain Res. 2012, 1454, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Ai, W.; Banik, N.L.; Ray, S.K. Overexpression of miR-7-1 increases efficacy of green tea polyphenols for induction of apoptosis in human malignant neuroblastoma SH-SY5Y and SK-N-DZ cells. Neurochem. Res. 2013, 38, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.W.; Yan, F.; Zhong, X.; Mazumder, P.B.; Xu-Monette, Z.Y.; Zou, D.; Young, K.H.; Ramos, K.S.; Li, Y. Regulation of p53-targeting MicroRNAs by polycyclic aromatic hydrocarbons: Implications in the etiology of multiple myeloma. Mol. Carcinog. 2015, 54, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Suarez, M.; Torres, J.L.; Salvado, M.J.; Arola, L.; Arola-Arnal, A. Resveratrol and EGCG bind directly and distinctively to miR-33a and miR-122 and modulate divergently their levels in hepatic cells. Nucleic Acids Res. 2014, 42, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, D.; Jude, B.; Morand, C. MiRNA as molecular target of polyphenols underlying their biological effects. Free Radic. Biol. Med. 2013, 64, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, J.; Zhang, J.; Miao, Q.; Yao, L.; Zhang, J. Curcumin promotes apoptosis by activating the p53-miR-192–5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015, 357, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fang, B.; Zeng, F.; Pang, H.; Zhang, J.; Shi, Y.; Wu, X.; Cheng, L.; Ma, C.; Xia, J.; et al. Curcumin inhibits cell growth and invasion through up-regulation of miR-7 in pancreatic cancer cells. Toxicol. Lett. 2014, 231, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Phuah, N.H.; Nagoor, N.H. Regulation of microRNAs by natural agents: New strategies in cancer therapies. BioMed Res. Int. 2014, 2014, 804510. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, S.; Thirumalai, K.; Danda, R.; Krishnakumar, S. Effect of curcumin on miRNA expression in human Y79 retinoblastoma cells. Curr. Eye Res. 2012, 37, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.D.; Jutooru, I.; Safe, S.; Angel-Morales, G.; Mertens-Talcott, S.U. The drug resistance suppression induced by curcuminoids in colon cancer SW-480 cells is mediated by reactive oxygen species-induced disruption of the microRNA-27A-ZBTB10-SP axis. Mol. Nutr. Food Res. 2013, 57, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, T.; Chen, C. The critical roles of miR-21 in anti-cancer effects of curcumin. Ann. Transl. Med. 2015, 3. [Google Scholar] [CrossRef]
- Subramaniam, D.; Ponnurangam, S.; Ramamoorthy, P.; Standing, D.; Battafarano, R.J.; Anant, S.; Sharma, P. Curcumin induces cell death in esophageal cancer cells through modulating notch signaling. PLoS ONE 2012, 7, e30590. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Dubaybo, H.; Ali, S.; Goncalves, P.; Kollepara, S.L.; Sethi, S.; Philip, P.A.; Li, Y. Down-regulation of miR-221 inhibits proliferation of pancreatic cancer cells through up-regulation of PTEN, p27(KIP1), p57(KIP2), and PUMA. Am. J. Cancer Res. 2013, 3, 465–477. [Google Scholar] [PubMed]
- Zhang, J.; Du, Y.; Wu, C.; Ren, X.; Ti, X.; Shi, J.; Zhao, F.; Yin, H. Curcumin promotes apoptosis in human lung adenocarcinoma cells through miR-186* signaling pathway. Oncol. Rep. 2010, 24, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, Y.; Sun, J.; Zhang, Y. Curcumin reduces the expression of BCL-2 by upregulating miR-15a and miR-16 in MCF-7 cells. Med. Oncol. 2010, 27, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Vislovukh, A.; Kratassiouk, G.; Porto, E.; Gralievska, N.; Beldiman, C.; Pinna, G.; El’skaya, A.; Harel-Bellan, A.; Negrutskii, B.; Groisman, I. Proto-oncogenic isoform A2 of eukaryotic translation elongation factor EEF1 is a target of miR-663 and miR-744. Br. J. Cancer 2013, 108, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liang, H.; Xia, Q.; Li, P.; Kong, H.; Lei, P.; Wang, S.; Tu, Z. Resveratrol induces apoptosis of pancreatic cancers cells by inhibiting miR-21 regulation of BCL-2 expression. Clin. Transl. Oncol. 2013, 15, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Chen, H.A.; Chen, P.S.; Cheng, Y.J.; Hsu, W.H.; Chang, Y.W.; Chen, Y.H.; Jan, Y.; Hsiao, M.; Chang, T.Y.; et al. miR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene 2013, 32, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Larrosa, M.; Yanez-Gascon, M.J.; Davalos, A.; Gil-Zamorano, J.; Gonzalvez, M.; Garcia-Almagro, F.J.; Ruiz Ros, J.A.; Tomas-Barberan, F.A.; Espin, J.C.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Hicks, C.; Levenson, A.S. Resveratrol and prostate cancer: Promising role for microRNAs. Mol. Nutr. Food Res. 2011, 55, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.J.; Adair, B.; Alder, H.; Limagne, E.; Taccioli, C.; Ferracin, M.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting junb and jund. Carcinogenesis 2010, 31, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.L.; Takami, A.; Trung, L.Q.; Kato, S.; Nakao, S. Resveratrol prevents EBV transformation and inhibits the outgrowth of EBV-immortalized human b cells. PLoS ONE 2012, 7, e51306. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Kumar, A.; Rimando, A.M.; Zhang, X.; Levenson, A.S. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget 2015, 6, 27214–27226. [Google Scholar] [CrossRef] [PubMed]
- Gil-Zamorano, J.; Martin, R.; Daimiel, L.; Richardson, K.; Giordano, E.; Nicod, N.; Garcia-Carrasco, B.; Soares, S.M.; Iglesias-Gutierrez, E.; Lasuncion, M.A.; et al. Docosahexaenoic acid modulates the enterocyte CACO-2 cell expression of microRNAs involved in lipid metabolism. J. Nutr. 2014, 144, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Han, C.; Song, K.; Zhang, J.; Lim, K.; Wu, T. Omega-3 polyunsaturated fatty acids upregulate 15-PGDH expression in cholangiocarcinoma cells by inhibiting miR-26A/B expression. Cancer Res. 2015, 75, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.C.; Chiang, E.P.; Tsai, S.Y.; Wang, F.Y.; Pai, M.H.; Syu, J.N.; Cheng, C.C.; Rodriguez, R.L.; Tang, F.Y. Eicosapentaenoic acid induces neovasculogenesis in human endothelial progenitor cells by modulating C-KIT protein and PI3-K/AKT/ENOS signaling pathways. J. Nutr. Biochem. 2014, 25, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Antal, O.; Hackler, L., Jr.; Shen, J.; Man, I.; Hideghety, K.; Kitajka, K.; Puskas, L.G. Combination of unsaturated fatty acids and ionizing radiation on human glioma cells: Cellular, biochemical and gene expression analysis. Lipids Health Dis. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.C.; Ghosh-Choudhury, T.; Dey, N.; Choudhury, G.G.; Ghosh-Choudhury, N. miR-21 is targeted by omega-3 polyunsaturated fatty acid to regulate breast tumor CSF-1 expression. Carcinogenesis 2012, 33, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Giordano, E.; Nicod, N.M.; Davalos, A. Molecular targets of omega 3 and conjugated linoleic fatty acids—“Micromanaging” cellular response. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- DIANA-miRPath v3.0. Available online: http://snf-515788.vm.okeanos.grnet.gr/dianauniverse/index.php?r=mirpath (accessed on 1 March 2016).
- Quinn, S.R.; O’Neill, L.A. A trio of microRNAs that control toll-like receptor signalling. Int. Immunol. 2011, 23, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Volkmann, I.; Thum, T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011, 8, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zou, F.; Zhang, X.; Li, H.; Dulak, A.; Tomko, R.J., Jr.; Lazo, J.S.; Wang, Z.; Zhang, L.; Yu, J. MicroRNA-21 negatively regulates CDC25A and cell cycle progression in colon cancer cells. Cancer Res. 2009, 69, 8157–8165. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.R.; Liu, X.J.; Li, X.J.; Shen, Z.Z.; Yang, B.; Wu, C.C.; Li, J.F.; Miao, L.F.; Ye, H.Q.; Qiao, G.H.; et al. MicroRNA miR-21 attenuates human cytomegalovirus replication in neural cells by targeting CDC25A. J. Virol. 2015, 89, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nangia-Makker, P.; Farhana, L.; Rajendra, S.G.; Levi, E.; Majumdar, A.P. miR-21 and miR-145 cooperation in regulation of colon cancer stem cells. Mol. Cancer 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Marques-Rocha, J.L.; Samblas, M.; Milagro, F.I.; Bressan, J.; Martinez, J.A.; Marti, A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 2015, 29, 3595–3611. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.J.; Wernicke, D.; Alder, H.; Costinean, S.; Volinia, S.; Croce, C.M. Mutator activity induced by microRNA-155 (miR-155) links inflammation and cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 4908–4913. [Google Scholar] [CrossRef] [PubMed]
- Dinami, R.; Ercolani, C.; Petti, E.; Piazza, S.; Ciani, Y.; Sestito, R.; Sacconi, A.; Biagioni, F.; le Sage, C.; Agami, R.; et al. miR-155 drives telomere fragility in human breast cancer by targeting TRF1. Cancer Res. 2014, 74, 4145–4156. [Google Scholar] [CrossRef] [PubMed]
- Smogorzewska, A.; de Lange, T. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 2004, 73, 177–208. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, W.; Miao, R.; Zhou, Y.; Wang, Z.; Zhang, L.; Wan, Y.; Dong, Y.; Qu, K.; Liu, C. miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FOXM1/C-myc pathway. Oncotarget 2015, 6, 3988–4004. [Google Scholar] [CrossRef] [PubMed]
- Mogilyansky, E.; Rigoutsos, I. The miR-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013, 20, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Halicka, H.D.; Zhao, H.; Li, J.; Lee, Y.S.; Hsieh, T.C.; Wu, J.M.; Darzynkiewicz, Z. Potential anti-aging agents suppress the level of constitutive MTOR- and DNA damage-signaling. Aging 2012, 4, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, Z.; Zhao, H.; Halicka, H.D.; Li, J.; Lee, Y.S.; Hsieh, T.C.; Wu, J.M. In search of antiaging modalities: Evaluation of MTOR- and ROS/DNA damage-signaling by cytometry. Cytometry A 2014, 85, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Pan, Y.; Navarro, F.; Dykxhoorn, D.M.; Moreau, L.; Meire, E.; Bentwich, Z.; Lieberman, J.; Chowdhury, D. miR-24-mediated downregulation of H2AX suppresses DNA repair in terminally differentiated blood cells. Nat. Struct. Mol. Biol. 2009, 16, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Halicka, H.D.; Huang, X.; Traganos, F.; Darzynkiewicz, Z. Constitutive histone H2AX phosphorylation and atm activation, the reporters of DNA damage by endogenous oxidants. Cell Cycle 2006, 5, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Yoshida, Y.; Suda, M.; Minamino, T. DNA damage response and metabolic disease. Cell Metab. 2014, 20, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Vose, S.; Mitchell, J. Relationship between DNA Damage and Energy Metabolism: Evidence from DNA Repair Deficiency Syndromes; INTECH Open Access Publisher: Rijeka, Croatia, 2011. [Google Scholar]
- Assaily, W.; Rubinger, D.A.; Wheaton, K.; Lin, Y.; Ma, W.; Xuan, W.; Brown-Endres, L.; Tsuchihara, K.; Mak, T.W.; Benchimol, S. ROS-mediated p53 induction of LPIN1 regulates fatty acid oxidation in response to nutritional stress. Mol. Cell 2011, 44, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; LaGory, E.L.; Kenzelmann Broz, D.; Bieging, K.T.; Brady, C.A.; Link, N.; Abrams, J.M.; Giaccia, A.J.; Attardi, L.D. Analysis of p53 transactivation domain mutants reveals ACAD11 as a metabolic target important for p53 pro-survival function. Cell Rep. 2015, 10, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. An emerging role of MTOR in lipid biosynthesis. Curr. Biol. 2009, 19, R1046–R1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, G.A. The integral role of mtor in lipid metabolism. Cell Cycle 2011, 10, 861–862. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Wu, P.; Senthilkumar, R.; Tian, X.; Liu, H.; Shen, X.; Tao, Z.; Huang, P. Loss of fatty acid synthase suppresses the malignant phenotype of colorectal cancer cells by down-regulating energy metabolism and MTOR signaling pathway. J. Cancer Res. Clin. Oncol. 2016, 142, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Flavin, R.; Peluso, S.; Nguyen, P.L.; Loda, M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010, 6, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Leontieva, O.V.; Demidenko, Z.N.; Blagosklonny, M.V. Dual MTORC1/C2 inhibitors suppress cellular geroconversion (a senescence program). Oncotarget 2015, 6, 23238–23248. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Rapamycin extends life and health span because it slows aging. Aging 2013, 5, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Jinka, R.; Kapoor, R.; Sistla, P.G.; Raj, T.A.; Pande, G. Alterations in cell-extracellular matrix interactions during progression of cancers. Int. J. Cell Biol. 2012, 2012, 219196. [Google Scholar] [CrossRef] [PubMed]
- Dickreuter, E.; Cordes, N. Cell-ECM interactions control DDR. Oncoscience 2015, 2, 679–680. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tanaka, T.; Mitlitski, V.; Heeter, J.; Balazs, E.A.; Darzynkiewicz, Z. Protective effect of hyaluronate on oxidative DNA damage in WI-38 and A549 cells. Int. J. Oncol. 2008, 32, 1159–1167. [Google Scholar] [PubMed]
- Darzynkiewicz, Z.; Balazs, E.A. Genome integrity, stem cells and hyaluronan. Aging 2012, 4, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Halicka, H.D.; Mitlitski, V.; Heeter, J.; Balazs, E.A.; Darzynkiewicz, Z. Attenuation of the oxidative burst-induced DNA damage in human leukocytes by hyaluronan. Int. J. Mol. Med. 2009, 23, 695–699. [Google Scholar] [PubMed]
- Gangaraju, V.K.; Lin, H. MicroRNAs: Key regulators of stem cells. Nat. Rev. Mol. Cell Biol. 2009, 10, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.D.; Venkatasubrahmanyam, S.; Jia, F.; Sun, N.; Butte, A.J.; Wu, J.C. MicroRNA profiling of human-induced pluripotent stem cells. Stem Cells Dev. 2009, 18, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Wu, J.C. MicroRNA expression profiling of human-induced pluripotent and embryonic stem cells. Methods Mol. Biol. 2013, 936, 247–256. [Google Scholar] [PubMed]
- Lei, H.; Quelle, F.W. Foxo transcription factors enforce cell cycle checkpoints and promote survival of hematopoietic cells after DNA damage. Mol. Cancer Res. 2009, 7, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. MTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, Y.D.; Wagers, A.J. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014, 20, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kim, H.W.; Matsu-Ura, K.; Wang, Y.G.; Xu, M.; Ashraf, M. Abrogation of age-induced microRNA-195 rejuvenates the senescent mesenchymal stem cells by reactivating telomerase. Stem Cells 2016, 34, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kong, X.; Lv, L.; Gao, J. TGF-β1 acts through miR-155 to down-regulate TP53INP1 in promoting epithelial-mesenchymal transition and cancer stem cell phenotypes. Cancer Lett. 2015, 359, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Puig, T.; Vazquez-Martin, A.; Relat, J.; Petriz, J.; Menendez, J.A.; Porta, R.; Casals, G.; Marrero, P.F.; Haro, D.; Brunet, J.; et al. Fatty acid metabolism in breast cancer cells: Differential inhibitory effects of epigallocatechin gallate (EGCG) and C75. Breast Cancer Res. Treat. 2008, 109, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Relat, J.; Blancafort, A.; Oliveras, G.; Cufi, S.; Haro, D.; Marrero, P.F.; Puig, T. Different fatty acid metabolism effects of (−)-epigallocatechin-3-gallate and C75 in adenocarcinoma lung cancer. BMC Cancer 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aljarbou, A.N.; Aldebasi, Y.H.; Faisal, S.M.; Khan, M.A. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014, 38, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Liang, Y.; Jiang, B.; Li, X.; Xun, H.; Sun, J.; He, W.; Lau, H.T.; Ma, X. Curcumin inhibits intracellular fatty acid synthase and induces apoptosis in human breast cancer MDA-MB-231 cells. Oncol. Rep. 2016, 35, 2651–2656. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Ho, K.A.; Brown, P.; Greenlee, H.; Bevers, T.B.; Arun, B.; Sneige, N.; Hudis, C.; McArthur, H.L.; Chang, J.; et al. Effects of a green tea extract, polyphenon e, on systemic biomarkers of growth factor signalling in women with hormone receptor-negative breast cancer. J. Hum. Nutr. Diet. 2015, 28, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase IIA clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Momchilova, A.; Petkova, D.; Staneva, G.; Markovska, T.; Pankov, R.; Skrobanska, R.; Nikolova-Karakashian, M.; Koumanov, K. Resveratrol alters the lipid composition, metabolism and peroxide level in senescent rat hepatocytes. Chem. Biol. Interact. 2014, 207, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Chan, Y.C.; Wang, M.F.; Wei, C.C.; Chang, S.J. Dietary (−)-epigallocatechin-3-gallate supplementation counteracts aging-associated skeletal muscle insulin resistance and fatty liver in senescence-accelerated mouse. J. Agric. Food Chem. 2015, 63, 8407–8417. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Shi, Y.; Tan, G.; Yang, C.H.; Fan, M.; Pfeffer, L.M.; Wu, Z.H. DNA damage induces NF-κB-dependent microRNA-21 up-regulation and promotes breast cancer cell invasion. J. Biol. Chem. 2012, 287, 21783–21795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wan, G.; Mlotshwa, S.; Vance, V.; Berger, F.G.; Chen, H.; Lu, X. Oncogenic WIP1 phosphatase is inhibited by miR-16 in the DNA damage signaling pathway. Cancer Res. 2010, 70, 7176–7186. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar]






| miRs | Reference |
|---|---|
| miR-34a/b/c, miR-192, miR-215, miR-16-1, miR-143, miR-107, let-7, miR-200c, miR-16, miR-145, miR-134, miR-449a/b, miR-503, miR-21, miR-24, miR-421, miR-504, miR-125b, miR-106b, miR-21, miR-210, miR-373, miR-100, miR-195, miR-124a, miR-290 cluster (miR-291-3p, miR-294, miR-295) | [5,27] |
| miR-363, miR-25, miR-542 | [28] |
| miR-421, miR-24, miR-34a/b/c, miR-504, miR-125b, miR-302, miR-92, miR-192, miR-194, miR-215, miR-106a-92 cluster (miR-106a, miR-18b, miR-20b, miR-19b-2, miR-92a-2, miR-363), miR-106b/25 cluster (miR-106b, miR-25, miR-93), miR-210, miR-128, miR-20, miR-130b, miR-143, miR-145, miR-16-1, miR-16, miR-103, miR-26a, miR-206 | [27] |
| miR-15a, miR-29, miR-107, miR-605, miR-17-92 cluster (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, miR-92a-1), miR-21-605, miR-221, miR-222, miR-138, miR-223, miR-181a, miR-27a, miR-214, miR-101, miR-185, miR-100, miR-506, miR-545, miR-124, miR-9, miR-182, miR-146a | [10] |
| Compound | miRNA | Reference | Cells/Cancer | Dose/Duration |
|---|---|---|---|---|
| EGCG | miR-18, miR-16, let-7a, miR-221, miR-34b, miR-193b, miR-222, miR-342 | [29] | hepatic cancer | 100 μM, 24 h |
| miR-636, miR-3907 | [30] | normal dermal fibroblasts | 10 μM, 24 h | |
| miR-200c | [31] | colorectal cancer cells and colon cancer stem cells | 100 μM, 24 h | |
| miR-210, miR-98-5p | [32,33] | lung cancer | 40 μM, 9 h 10 μM, 24 h | |
| miR-1, miR-126 | [34,35] | osteosarcoma | 0.08 g/L, 48 h 0.2 g/L, 72 h | |
| miR-194 | [36] | hepatocarcinoma | 10 μg/mL, 48 h | |
| miR-7-1, miR-34a, miR-99a, miR-92, miR-93, miR-106b | [37,38] | neuroblastoma | 50 μM, 24 h | |
| miR-25, miR-92, miR-141, miR-200a | [39] | Hela cells and lymphoblasts | 1–5 μM, 24 h | |
| miR-33a, miR-122 | [40] | hepatocarcinoma | 50 μM, 1 h | |
| EGCG | miR-92, miR-93, miR-106b, miR-7-1, miR-34a, miR-99a | [41] | neuroblastoma | 50 μM, 24 h |
| miR-467bn, miR-487b, miR-197, miR-805, miR-374n, let-7f, miR-350, miR-24-1n, miR-137, miR-335-3p, let-7a, miR-222, miR-26b, miR-30c-1n, let-7d, miR-98, miR-30c, miR-30bn, miR-32, miR-674n, miR-532-5p, let-7g, miR-18a, miR-192, miR-302d, miR-30b, miR-802, let-7e, miR-322, miR-720, miR-146b, miR-340-3p, miR-185, miR-425, miR-10a, miR-126-5p, miR-101a, miR-30en, let-7c, miR-141, miR-33, miR-29an, miR-199b, miR-450a-5p, miR-21, miR-23a, miR-101b, miR-148a, miR-193, miR-23b, miR-107, miR-140, miR-551b, miR-466c-5p, miR-106a, miR-590-3p, miR-875-3p, miR-224, miR-292-5p, miR-678, miR-469, let-7bn, miR-463n, miR-574-3p, miR-201, miR-290-3p, miR-181a, miR-302a, miR-429, miR-133a, miR-190b, miR-710, miR-135b, miR-296-5p, miR-191n, miR-188-5p, miR-298, miR-181a-1n, miR-466g, miR-26bn, miR-466f-3p, miR-29bn, miR-1224, miR-291b-5p, miR-324-5p, miR-486, miR-128, miR-450b-3p, miR-135an, miR-294, miR-671-5p, miR-878-3p, miR-801, miR-370, miR-1, miR-494, miR-133b | [41] | hepatocarcinoma | 100 μM, 24 h | |
| CRC | miR-192-5p/215 | [42] | lung cancer | 15 μM, 48 h |
| miR-7 | [43] | pancreatic cancer | 3–6 μM, 72 h | |
| miR-22 | [44,45] | retinoblastoma | 20 μM, 48 h | |
| miR-27a | [46] | colon cancer | 2.5–10 μg/mL, 24 h | |
| miR-21 | [47] | |||
| let-7a, miR-21, miR-34a | [48] | esophageal cancer | 30 μM, 24 h | |
| miR-221 | [44,49] | pancreatic cancer | 500 nM of synthetic CRC analogue, 72 h | |
| CRC | miR-27a, miR-20a, miR-17-5p, miR-21 | [41] | colon carcinoma | 30 μM, 24 h |
| miR-203 | [41] | bladder carcinoma | 10 μM, 3 days | |
| miR-320, miR-26a, let-7i, miR-130a, miR-16, miR-125b, miR-23a, miR-27b, miR-155, miR-625, miR-576-3p, miR-186n, miR-9n, let-7i | [50] | lung adenocarcinoma | 15 μM, 48 h | |
| miR-15a, miR-16-1 | [41] | leukemic cells | 5–20 μM, 24-72 h | |
| miR15a, miR-16 | [51] | breast cancer | 10–60 μM, 24 h | |
| miR-103, miR-140, miR-146b, miR-148a, miR-15b, miR-181a, miR-181b, miR-181d, miR-195, miR-196a, miR-199an, miR-19a, miR-204, miR-20a, miR-21, miR-22, miR-23a, miR-23b, miR-24, miR-25, miR-26a, miR-27a, miR-34a, miR-374, miR-510, miR-7, miR-92, miR-93, miR-98 | [41] | pancreatic cancer | 10 μM, 72 h | |
| RSV | miR-663, miR-744m | [44,52] | breast cancer | 100 μM, 24 h |
| miR-21 | [44,53] | pancreatic cancer | 50 μM, 24 h | |
| miR-520h | [54] | lung cancer | 10–20 μM, 48 h | |
| miR-21, miR-181b, miR-663, miR-30c2 | [55] | peripheral blood mononuclear cells from hypertensive patients | RSV (8 mg) grape extract, one year daily intake ( in vivo study) | |
| miR-150, miR-296-5p | [56] | lymph node cancer prostate | 50 μM, 24 h | |
| miR-33a, miR-122 | [40] | hepatocarcinoma | 50 μM, 1 h | |
| miR-155 miR-663 | [57] | monocytic cells | 30–50 μM, 14 h | |
| RSV | miR-155, miR-34a | [58] | EBV-immortalized B cells | 25–50 μM, 24 h |
| miR-7, miR-17, miR-18b, miR-20a, miR-20b, miR-92b, miR-106a, miR106b, miR-17-5p, miR-20a, miR-106b, miR-17-92 cluster, miR-106ab clusters | [59] | prostate cancer | 50–100 μM, 24 h | |
| miR-622 | [41] | bronchial epithelial cells | 50 μM, 48 h | |
| miR-155, miR-633 | [41] | monocytes | 30 μM, 14 h | |
| let-7c, miR-106a, miR-106b, miR-1224-5p, miR-1228, miR-231, miR-1246, miR-1260, miR-1267, miR-1268, miR-129, miR-1290, miR-1308, miR-1469, miR-149, miR-150, miR-152, miR-15a, miR-17, miR-1825, miR-185, miR-18b, miR-1908, miR-1915, miR-197, miR-1972, miR-1973, miR-1974, miR-1975, miR-1977, miR-1979, miR-20a, miR-20b, miR-24, miR-296-5p, miR-483-5p, miR-513a-5p, miR-548q, miR-572, miR-575, miR-612, miR-638, miR-654-5p, miR-659, miR-671-5p, miR-7, miR-762, miR-764, miR-874, miR-92b, miR-939 | [41] | lymph node cancer prostate | 50 μM, 48 h | |
| miR-1, miR-100-1/2, miR-102, miR-103-1, miR-103-2, miR-146a, miR-146b-5p, miR-16-0, miR-17, miR-181a2, miR-194-2, miR-196a1, miR-205, miR-206, miR-21, miR-23a, miR-23b, miR-25, miR-26a, miR-29c, miR-30a-3p, miR-30c-1, miR-30d, miR-30e-5p, miR-323, miR-340, miR-363n-5p, miR-424, miR-494, miR-497, miR-560, miR-560, miR-565, miR-565, miR-572, miR-574, miR-594, miR-615, miR-622, miR-629, miR-631, miR-638, miR-639, miR-657, miR-659, miR-663, miR-801, miR-92a-2 | [41] | colorectal carcinoma | 50 μM, 14 h | |
| n3-PUFA | miR-192, miR-30c, miR-141-3p, miR-221-3p, miR-1283, let-7f, miR-181a-5p, miR-1, miR-30a | [60] | Caco-2 cells | 200 μM DHA in lipid micelles, 24 h |
| n3-PUFA | miR-26a, miR-26b | [61] | cholangiocarcinoma | 50 μM DHA, 12 h |
| miR-221 | [62] | endothelial progenitor cells | 25–125 μM EPA, 4 h | |
| miR-146, miR-181a | [63] | glioma | 25–50 μM DHA, 48 h | |
| miR-21 | [64] | breast cancer | 152 nM DHA, 24 h | |
| miR-30c, miR-20b, miR-16, miR-22, miR-145, miR-34, miR-25, miR-17, miR-26a, miR-29c, miR-200a, miR-206, miR-323, miR-16, miR-22, miR-20b, miR-30c, miR-183, miR-224, miR-145, miR-181a, miR-208, miR-143, miR-20a, miR-149, miR-125b | [19,65] | glioma | 50–100 μM DHA, 24 h |
| KEGG Pathway | p-Value | #of Genes | miRNAs |
|---|---|---|---|
| Fatty acid biosynthesis | 0 | 4 | miR-16 |
| Fatty acid metabolism | 2.23 × 10-5 | 7 | miR-16 |
| Thyroid hormone synthesis | 2.58 × 10-5 | 5 | miR-146b |
| Signaling pathways regulating pluripotency of stem cells | 0.0002346931 | 24 | miR-16 |
| Glioma | 0.000556152 | 15 | miR-16 miR-34a |
| Glycosphingolipid biosynthesis: lacto and neolacto series | 0.001440903 | 2 | miR-34a |
| Hippo signaling pathway | 0.00410767 | 24 | miR-16 miR-21 |
| Steroid hormone biosynthesis | 0.009497578 | 1 | miR-25 |
| Ovarian steroidogenesis | 0.01398717 | 1 | miR-25 |
| Melanoma | 0.01891852 | 14 | miR-16 |
| Prostate cancer | 0.02681897 | 16 | miR-16 |
| Cytokine-cytokine receptor interaction | 0.03260184 | 11 | miR-21 |
| mTOR signaling pathway | 0.03417768 | 13 | miR-16 miR-25 |
| Oocyte meiosis | 0.03995201 | 16 | miR-16 |
| KEGG Pathway | p-Value | # of Genes | # of miRNAs |
|---|---|---|---|
| Fatty acid biosynthesis | 0 | 4 | 3 |
| ECM-receptor interaction | 0 | 42 | 13 |
| Signaling pathways regulating pluripotency of stem cells | 2.44 × 10−9 | 76 | 17 |
| Amebiasis | 3.53 × 10−6 | 24 | 6 |
| Proteoglycans in cancer | 1.17 × 10−4 | 117 | 12 |
| Mucin type O-glycan biosynthesis | 1.67 × 10−2 | 15 | 10 |
| Glioma | 1.43 × 10−1 | 39 | 10 |
| TGF-β signaling pathway | 1.85 × 100 | 40 | 8 |
| Fatty acid metabolism | 2.28 × 101 | 9 | 3 |
| Focal adhesion | 0.0002057474 | 110 | 7 |
| PI3K-Akt signaling pathway | 0.001829865 | 138 | 7 |
| Lysine degradation | 0.006232265 | 18 | 7 |
| ErbB signaling pathway | 0.02315742 | 45 | 7 |
| Protein digestion and absorption | 0.02773474 | 26 | 3 |
| Thyroid hormone signaling pathway | 0.03049808 | 57 | 8 |
| Glycosaminoglycan biosynthesis heparan sulfate/heparin | 0.05012798 | 9 | 5 |
| KEGG Pathway | p-Value | # of Genes | # of miRNAs |
|---|---|---|---|
| Fatty acid biosynthesis | 0 | 4 | 5 |
| ECM-receptor interaction | 0 | 19 | 7 |
| Fatty acid metabolism | 6.88 × 10−9 | 8 | 4 |
| Signaling pathways regulating pluripotency of stem cells | 2.16 × 10−5 | 55 | 9 |
| Glioma | 0.0002249224 | 20 | 5 |
| Proteoglycans in cancer | 0.0003169881 | 66 | 6 |
| TGF-β signaling pathway | 0.006985672 | 28 | 5 |
| Prostate cancer | 0.009151789 | 31 | 5 |
| Axon guidance | 0.01011865 | 45 | 3 |
| Melanoma | 0.01304415 | 20 | 4 |
| Prolactin signaling pathway | 0.01811186 | 28 | 5 |
| Pathways in cancer | 0.0206275 | 70 | 4 |
| Glycosaminoglycan biosynthesis-heparan sulfate/heparin | 0.02616968 | 3 | 5 |
| KEGG Pathway | p-Value | # of Genes | # of miRNAs |
|---|---|---|---|
| Fatty acid biosynthesis | 0 | 5 | 7 |
| Fatty acid metabolism | 0 | 16 | 8 |
| Signaling pathways regulating pluripotency of stem cells | 1.07 × 10−6 | 64 | 13 |
| TGF-β signaling pathway | 1.39 × 10−4 | 34 | 9 |
| Proteoglycans in cancer | 8.1 × 10−1 | 70 | 10 |
| Axon guidance | 0.0009836851 | 55 | 6 |
| Hippo signaling pathway | 0.001005551 | 43 | 9 |
| Mucin type O-glycan biosynthesis | 0.002219981 | 12 | 8 |
| Glycosphingolipid biosynthesis: lacto and neolacto series | 0.006851962 | 6 | 7 |
| GABAergic synapse | 0.007317003 | 13 | 8 |
| Glioma | 0.03097475 | 21 | 7 |
| KEGG Pathway | p-Value | # of Genes | # of miRNAs |
|---|---|---|---|
| ECM-receptor interaction | 0 | 27 | 4 |
| Fatty acid biosynthesis | 5.57 × 10-5 | 4 | 1 |
| Glycosphingolipid biosynthesis: lacto and neolacto series | 2.21 × 10-2 | 8 | 8 |
| Mucin type O-glycan biosynthesis | 0.0003249162 | 9 | 5 |
| Proteoglycans in cancer | 0.0004576113 | 76 | 8 |
| TGF-β signaling pathway | 0.001807704 | 27 | 5 |
| Thyroid hormone synthesis | 0.002952912 | 5 | 3 |
| Amebiasis | 0.00834241 | 24 | 2 |
| Signaling pathways regulating pluripotency of stem cells | 0.02548584 | 51 | 4 |
| Glioma | 0.02578235 | 31 | 6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carotenuto, F.; Albertini, M.C.; Coletti, D.; Vilmercati, A.; Campanella, L.; Darzynkiewicz, Z.; Teodori, L. How Diet Intervention via Modulation of DNA Damage Response through MicroRNAs May Have an Effect on Cancer Prevention and Aging, an in Silico Study. Int. J. Mol. Sci. 2016, 17, 752. https://doi.org/10.3390/ijms17050752
Carotenuto F, Albertini MC, Coletti D, Vilmercati A, Campanella L, Darzynkiewicz Z, Teodori L. How Diet Intervention via Modulation of DNA Damage Response through MicroRNAs May Have an Effect on Cancer Prevention and Aging, an in Silico Study. International Journal of Molecular Sciences. 2016; 17(5):752. https://doi.org/10.3390/ijms17050752
Chicago/Turabian StyleCarotenuto, Felicia, Maria C. Albertini, Dario Coletti, Alessandra Vilmercati, Luigi Campanella, Zbigniew Darzynkiewicz, and Laura Teodori. 2016. "How Diet Intervention via Modulation of DNA Damage Response through MicroRNAs May Have an Effect on Cancer Prevention and Aging, an in Silico Study" International Journal of Molecular Sciences 17, no. 5: 752. https://doi.org/10.3390/ijms17050752
APA StyleCarotenuto, F., Albertini, M. C., Coletti, D., Vilmercati, A., Campanella, L., Darzynkiewicz, Z., & Teodori, L. (2016). How Diet Intervention via Modulation of DNA Damage Response through MicroRNAs May Have an Effect on Cancer Prevention and Aging, an in Silico Study. International Journal of Molecular Sciences, 17(5), 752. https://doi.org/10.3390/ijms17050752