Mitochondria Synthesize Melatonin to Ameliorate Its Function and Improve Mice Oocyte’s Quality under in Vitro Conditions
Abstract
:1. Introduction
2. Results
2.1. Oocytes Synthesize Melatonin during Maturation
2.2. Mitochondria are the Major Sites for Melatonin Synthesis in Oocytes
2.3. Melatonin Improves Oocytes Quality, and Accelerates IVF Embryo Development
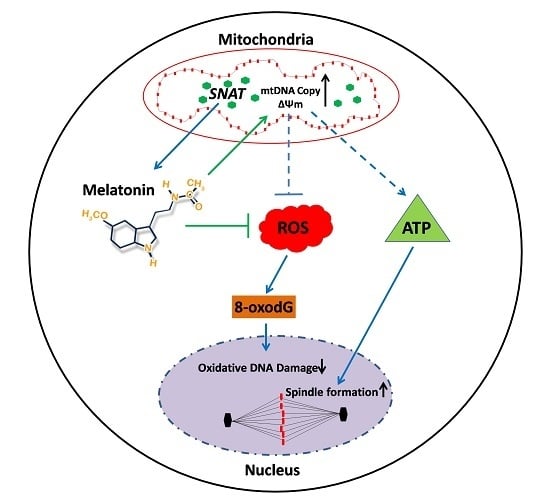
2.4. Melatonin Ameliorates the Function of Mitochondria
2.5. Melatonin Promotes Meiotic Spindle Assembly
2.6. Melatonin Protects Oocyte from Oxidative Damage
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animal Treatment
4.3. Analyses of Melatonin Receptors and Rate-Limiting Enzyme for Melatonin Synthesis
4.4. Immuno-Electron Microscopic Identification of Melatonin Synthesizing Sites
4.5. Melatonin Assay with High Performance Liquid Chromatography (HPLC)
4.6. Mitochondrial Isolation and Detection of Melatonin in Culture Medium
4.7. Western Blot Analysis
4.8. Oocyte in Vitro Maturation (IVM), in Vitro Fertilization (IVF) and Embryo Culture
4.9. Detection of mtDNA Copy Number by qPCR
4.10. Oocytes Mitochondrial Distribution Assay
4.11. Detection of Mitochondrial Membrane Potential (ΔΨm) and ATP Levels in Oocytes
4.12. Spindles Analysis
4.13. Measurement of Reactive Oxygen Species (ROS) and Glutathione (GSH)
4.14. 8-oxodG Assay
4.15. Gene Expression Assay with Reverse Transcriptional PCR or Real-Time qPCR
4.16. Statistics Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gilchrist, R.B.; Thompson, J.G. Oocyte maturation: Emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 2007, 67, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Krisher, R.L. In vivo and in vitro environmental effects on mammalian oocyte quality. Annu. Rev. Anim. Biosci. 2013, 1, 393–417. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Albertini, D.F. Regulation of meiotic maturation in the mammalian oocyte: Inteplay between exogenous cues and the microtubule cytoskeleton. Bioessays 1992, 14, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Su, Y.; Ariga, M.; Law, E.; Jin, S.C.; Conti, M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 2004, 303, 682–684. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Q.; Wu, X.; O’Brien, M.J.; Pendola, F.L.; Denegre, J.N.; Matzuk, M.M.; Eppig, J.J. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte–cumulus cell complex in mice: Genetic evidence for an oocyte–granulosa cell regulatory loop. Dev. Biol. 2004, 276, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.X.; Gilchrist, R.B.; Thompson, J.G.; Lane, M. Exogenous growth differentiation factor 9 in oocyte maturation media enhances subsequent embryo development and fetal viability in mice. Hum. Reprod. 2008, 23, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Su, Y.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.W.; Zhang, M.; Shuhaibar, L.C.; Norris, R.P.; Geerts, A.; Wunder, F.; Eppig, J.J.; Potter, L.R.; Jaffe, L.A. Luteinizing hormone reduces the activity of the NPR2 guanylyl cyclase in mouse ovarian follicles, contributing to the cyclic GMP decrease that promotes resumption of meiosis in oocytes. Dev. Biol. 2012, 366, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, D.; Albertini, D.F. Cell cycle control during mammalian oogenesis. Curr. Top. Dev. Biol. 1993, 28, 126–126. [Google Scholar]
- Su, Y.Q.; Sugiura, K.; Sun, F.; Pendola, J.K.; Cox, G.A.; Handel, M.A.; Schimenti, J.C.; Eppig, J.J. MARF1 regulates essential oogenic processes in mice. Science 2012, 335, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Q.; Sun, F.; Handel, M.A.; Schimenti, J.C.; Eppig, J.J. Meiosis arrest female 1 (MARF1) has nuage-like function in mammalian oocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 18653–18660. [Google Scholar] [CrossRef] [PubMed]
- Espey, L.L. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol. Reprod. 1994, 50, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Russell, D.L.; Ochsner, S.; Espey, L.L. Ovulation: New dimensions and new regulators of the inflammatory-like response. Annu. Rev. Physiol. 2002, 64, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Behrman, H.R.; Kodaman, P.H.; Preston, S.L.; Gao, S. Oxidative stress and the ovary. J. Soc. Gynecol. Investig. 2001, 8, S40–S42. [Google Scholar] [CrossRef]
- Carbone, M.C.; Tatone, C.; Delle Monache, S.; Marci, R.; Caserta, D.; Colonna, R.; Amicarelli, F. Antioxidant enzymatic defences in human follicular fluid: Characterization and age-dependent changes. Mol. Hum. Reprod. 2003, 9, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Revelli, A.; Delle Piane, L.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 4330–4337. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal. Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Maldonado, M.D. Melatonin as an antioxidant: Physiology versus pharmacology. J. Pineal. Res. 2005, 39, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.H.C.; Leal, C.L.V.; da Cruz, J.F.; Tan, D.; Reiter, R.J. Role of melatonin on production and preservation of gametes and embryos: A brief review. Anim. Reprod. Sci. 2014, 145, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Dair, E.L.; Simoes, R.S.; Simões, M.J.; Romeu, L.R.G.; Oliveira-Filho, R.M.; Haidar, M.A.; Baracat, E.C.; Soares, J.M. Effects of melatonin on the endometrial morphology and embryo implantation in rats. Fertil. Steril. 2008, 89, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- He, C.J.; Wang, J.; Li, Y.; Zhu, K.F.; Xu, Z.Y.; Song, Y.L.; Song, Y.K.; Liu, G.S. Melatonin-related genes expressed in the mouse uterus during early gestation promote embryo implantation. J. Pineal. Res. 2015, 58, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Yellon, S.M.; Longo, L.D. Melatonin rhythms in fetal and maternal circulation during pregnancy in sheep. Am. J. Physiol.-Endoc. Metab. 1987, 252, 799–802. [Google Scholar]
- Brzezinski, A.; Seibel, M.M.; Lynch, H.J.; Deng, M.; Wurtman, R.J. Melatonin in human preovulatory follicular fluid. J. Clin. Endocr. Metab. 1987, 64, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Tamura, H.; Takayama, H.; Kato, H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil. Steril. 2003, 80, 1012–1016. [Google Scholar] [CrossRef]
- Fu, Y.; He, C.J.; Ji, P.Y.; Zhuo, Z.Y.; Tian, X.Z.; Wang, F.; Tan, D.X.; Liu, G.S. Effects of melatonin on the proliferation and apoptosis of Sheep Granulosa Cells under Thermal Stress. Int. J. Mol. Sci. 2014, 15, 21090–21104. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Yie, S.M.; Niles, L.P.; Younglai, E.V. Melatonin receptors on human granulosa cell membranes. J. Clin. Endocr. Metab. 1995, 80, 1747–1749. [Google Scholar] [PubMed]
- Woo, M.M.; Tai, C.; Kang, S.K.; Nathwani, P.S.; Pang, S.F.; Leung, P.C. Direct action of melatonin in human granulosa-luteal cells. J. Clin. Endocr. Metab. 2001, 86, 4789–4797. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.; Itoh, M.T.; Takahashi, N.; Tarumi, W.; Ishizuka, B. The rat oocyte synthesises melatonin. Reprod. Fertil. Dev. 2013, 25, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological implications. Fertil. Steril. 2009, 92, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; Sugino, N. Melatonin and female reproduction. Obstet. Gynaecol. Res. 2014, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.M.; Tian, X.Z.; Zhou, G.B.; Wang, L.; Gao, C.; Zhu, S.E.; Zeng, S.M.; Tian, J.H.; Liu, G.S. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J. Pineal Res. 2009, 47, 318–323. [Google Scholar] [CrossRef] [PubMed]
- El-Raey, M.; Geshi, M.; Somfai, T.; Kaneda, M.; Hirako, M.; Abdel Ghaffar, A.E.; Sosa, G.A.; El Roos, M.E.; Nagai, T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev. 2011, 78, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Wurtman, R.J.; Alonso, R.; Lynch, H.J. Melatonin secretion decreases during the proestrous stage of the rat estrous cycle. Proc. Natl. Acad. Sci. USA 1978, 75, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J.; Axelrod, J.; Potter, L.T. The uptake of H3-melatonin in endocrine and nervous tissues and the effects of constant light exposure. J. Pharmacol. Exp. Ther. 1964, 143, 314–318. [Google Scholar] [PubMed]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales Corral, S.A.; Acuna Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondria dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Van Blerkom, J.; Davis, P.; Mathwig, V.; Alexander, S. Domains of high-polarized and low-polarized mitochondria may occurin mouse and human oocytes and early embryos. Hum. Reprod. 2002, 17, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Z.; Wang, F.; He, C.J.; Zhang, L.; Tan, D.X.; Reiter, R.J.; Xu, J.; Ji, P.Y.; Liu, G.S. Beneficial effects of melatonin on bovine oocytes maturation: A mechanistic approach. J. Pineal Res. 2014, 57, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.J.; Peng, T.I.; Yu, P.Z.; Jou, S.B.; Reiter, R.J.; Chen, J.Y.; Wu, H.Y.; Chen, C.C.; Hsu, L.F. Melatonin protects against common deletion of mitochondria DNA-augmented mitochondria oxidative stress and apoptosis. J. Pineal Res. 2007, 43, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wang, Z.; An, L.; Zhang, Z.; Tan, K.; Miao, K.; Tao, L.; Cheng, L.; Zhang, Z.; Yang, M.; et al. Dynamic comparisons of high-resolution expression profiles highlighting mitochondria-related genes between in vivo and in vitro fertilized early mouse embryos. Hum. Reprod. 2015, 30, 2892–2911. [Google Scholar] [PubMed]
- Semak, I.; Naumova, M.; Korik, E.; Terekhovich, V.; Wortsman, J.; Slominski, A. A novel metabolic pathway of melatonin: Oxidation by cytochrome C. Biochemistry 2005, 44, 9300–9307. [Google Scholar] [CrossRef] [PubMed]
- Yamochi, T.; Hashimoto, S.; Amo, A.; Goto, H.; Yamanaka, M.; Inoue, M.; Nakaoka, Y.; Morimoto, Y. Mitochondrial dynamics and their intracellular traffic in porcine oocytes. Zygote 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Mabuchi, T.; Hirata, S.; Shoda, T.; Kasai, T.; Yokota, S.; Hoshi, K. Correlation of abnormal mitochondrial distribution in mouse oocytes with reduced developmental competence. Tohoku Jexpmed 2006, 210, 137–144. [Google Scholar] [CrossRef]
- Brevini, T.A.L.; Cillo, F.; Antonini, S.; Gandolfi, F. Cytoplasmic remodelling and the acquisition of developmental competence in pig oocytes. Anim. Reprod. Sci. 2007, 98, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Bavister, B.D.; Squirrell, J.M. Mitochondrial distribution and function in oocytes and early embryos. Hum. Reprod. 2000, 15, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, M.; Rottenberg, H. Relation between the gradient of the ATP/ADP ratio and the membrane potential across the mitochondrial membrane. Eur. J. Biochem. 1977, 73, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Selesniemi, K.; Lee, H.; Muhlhauser, A.; Tilly, J.L. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc. Natl. Acad. Sci. USA 2011, 108, 12319–12324. [Google Scholar] [CrossRef] [PubMed]
- Thouas, G.A.; Trounson, A.O.; Wolvetang, E.J.; Jones, G.M. Mitochondria dysfunction in mouse oocytes results in preimplantation embryo arrest in vitro. Biol. Reprod. 2004, 71, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- McNally, F.J. Mechanisms of spindle positioning. J. Cell Biol. 2013, 200, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Bormann, C.L.; Ongeri, E.M.; Krisher, R.L. The effect of vitamins during maturation of caprine oocytes on subsequent developmental potential in vitro. Theriogenology 2003, 59, 1373–1380. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.Z.; Zhang, L.; He, C.J.; Ji, P.Y.; Li, Y.; Tan, D.X.; Liu, G.S. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil. Steril. 2014, 101, 577–586. [Google Scholar] [CrossRef] [PubMed]
- García Ruiz, C.; Morales, A.; Colell, A.; Ballesta, A.; Rodés, J.; Kaplowitz, N.; Fernández Checa, J.C. Feeding S-adenosyl-l-methionine attenuates both ethanol-induced depletion of mitochondria glutathione and mitochondria dysfunction in periportal and perivenous rat hepatocytes. Hepatology 1995, 21, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.; Koolmeister, T.; Jemth, A.; Eshtad, S.; Jacques, S.A.; Ström, C.E.; Svensson, L.M.; Schultz, N.; Lundbäck, T.; Einarsdottir, B.O. MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature 2014, 508, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of Caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Rossé, T.; Olivier, R.; Monney, L.; Rager, M.; Conus, S.; Fellay, I.; Jansen, B.; Borner, C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature 1998, 391, 496–499. [Google Scholar] [PubMed]
- Zhao, Y.; Tan, D.X.; Lei, Q.; Chen, H.; Wang, L.; Li, Q.T.; Gao, Y.; Kong, J. Melatonin and its potential biological functions in the fruits of sweet cherry. J. Pineal Res. 2013, 55, 79–88. [Google Scholar] [CrossRef] [PubMed]








| Genes | Primer Sequence(5’–3’) | Tm(°C) |
|---|---|---|
| β-Actin | Forward: CCAGCCTTCCTTCTTGGGTAT | 60 |
| Reverse: AGGTCTTTACGGATGTCAACG | ||
| p53 | Forward: TGAGGTTCGTGTTTGTGCCTGC | 60 |
| Reverse: CCATCAAGTGGTTTTTTCTTTTGC | ||
| Bcl-2 | Forward: ACCTGTGGTCCATCTGACCCTC | 60 |
| Reverse: CCAGTTCACCCCATCCCTGA | ||
| Caspase-3 | Forward: CTGGAGAAATTCAAAGGACGGG | 60 |
| Reverse: TGAGCATGGACACAATACACGG | ||
| MT-ND5 | Forward: ATAGCCTGGCAGACGAACAAGACA | 60 |
| Reverse: AATTAGTAGGGCTCAGGCGTTGGT | ||
| MT1 | Forward: CCATTTCATCGTGCCTATG | 58 |
| Reverse: GTAACTAGCCACGAACAGC | ||
| MT2 | Forward: TACATCAGCCTCGTCTGGCTCC | 58 |
| Reverse: TTCCTCGTAGCCTTGGCCTTCC | ||
| SNAT | Forward: TGAACATCAACTCCCTGAAACCT | 60 |
| Reverse: TTCCCGCTCAATCTCAAACG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria Synthesize Melatonin to Ameliorate Its Function and Improve Mice Oocyte’s Quality under in Vitro Conditions. Int. J. Mol. Sci. 2016, 17, 939. https://doi.org/10.3390/ijms17060939
He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, Ma T, Tao J, Zhu K, Song Y, et al. Mitochondria Synthesize Melatonin to Ameliorate Its Function and Improve Mice Oocyte’s Quality under in Vitro Conditions. International Journal of Molecular Sciences. 2016; 17(6):939. https://doi.org/10.3390/ijms17060939
Chicago/Turabian StyleHe, Changjiu, Jing Wang, Zhenzhen Zhang, Minghui Yang, Yu Li, Xiuzhi Tian, Teng Ma, Jingli Tao, Kuanfeng Zhu, Yukun Song, and et al. 2016. "Mitochondria Synthesize Melatonin to Ameliorate Its Function and Improve Mice Oocyte’s Quality under in Vitro Conditions" International Journal of Molecular Sciences 17, no. 6: 939. https://doi.org/10.3390/ijms17060939
APA StyleHe, C., Wang, J., Zhang, Z., Yang, M., Li, Y., Tian, X., Ma, T., Tao, J., Zhu, K., Song, Y., Ji, P., & Liu, G. (2016). Mitochondria Synthesize Melatonin to Ameliorate Its Function and Improve Mice Oocyte’s Quality under in Vitro Conditions. International Journal of Molecular Sciences, 17(6), 939. https://doi.org/10.3390/ijms17060939