Enhancement of Matrix Metalloproteinase-2 (MMP-2) as a Potential Chondrogenic Marker during Chondrogenic Differentiation of Human Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Results
2.1. Characterization of hASCs
2.2. Proteolytic Enzyme Activity in hASCs during Differentiation
2.3. Chondrogenic Differentiation of hASCs on Col II-Coated Plates
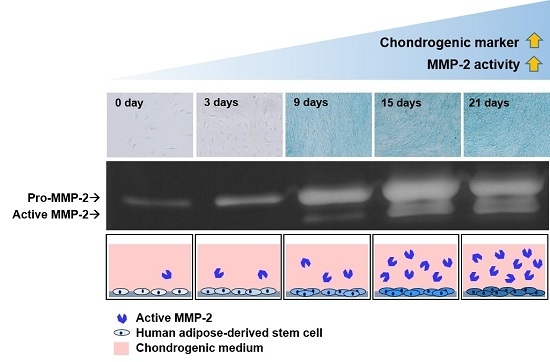
2.4. MMP-2 and Hypertrophic Marker Genes Expression of hASCs during Chondrogenic Differentiation in the Presence of Col II
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of hASCs
4.2. Differentiation of hASCs
4.3. Proliferation and Migration of hASCs
4.4. Measurement of Proteolytic Enzyme Activity
4.5. Zymography
4.6. Quantitative Real-Time PCR
4.7. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ad-ASC | hASCs undergoing adipogenic differentiation |
| ALP | Alkaline phosphatase |
| AM | Adipogenic medium |
| C/EBPβ | CCAAT/enhancer binding protein beta |
| CCK-8 | Cell counting kit-8 |
| Cd-ASC | hASCs undergoing chondrogenic differentiation |
| CM | Chondrogenic medium |
| Col I | Type I collagen |
| Col II | Type II collagen |
| Col X | Type X collagen |
| DEX | Dexamethasone |
| DQ-BSA | Dye quenched-bovine serum albumin |
| ECM | Extracellular matrix |
| GAG | Glycosminoglycan |
| hASCs | Human adipose-derived stem cells |
| MMP-13 | Matrix metalloproteinase 13 |
| MMPs | Matrix metalloproteinases |
| MSCs | Mesenchymal stem cells |
| Od-ASC | hASCs undergoing osteogenic differentiation |
| OM | Osteogenic medium |
| PPARγ | Peroxisome proliferator activated receptor gamma |
| RPS18 | Ribosomal protein S18 |
| RUNX2 | Runt-related transcription factor 2 |
| Sox9 | Sex determining region Y-box9 |
| TGF | Transforming growth factor |
| Ud-ASC | Undifferentiating hASCs |
References
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Petit, I. Current understanding of stem cell mobilization: The roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 2002, 30, 973–981. [Google Scholar] [CrossRef]
- Kasper, G.; Glaeser, J.D.; Geissler, S.; Ode, A.; Tuischer, J.; Matziolis, G.; Perka, C.; Duda, G.N. Matrix metalloprotease activity is an essential link between mechanical stimulus and mesenchymal stem cell behavior. Stem Cells 2007, 25, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.H.; Werb, Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 2000, 14, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Paiva, K.B.; Granjeiro, J.M. Bone tissue remodeling and development: Focus on matrix metalloproteinase functions. Arch. Biochem. Biophys. 2014, 561, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, G. Roles of matrix metalloproteinase in migration and differentiation of bone marrow-derived mesenchymal stem cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2012, 29, 387–391. [Google Scholar] [PubMed]
- Mosig, R.A.; Dowling, O.; DiFeo, A.; Ramirez, M.C.; Parker, I.C.; Abe, E.; Diouri, J.; Aqeel, A.A.; Wylie, J.D.; Oblander, S.A.; et al. Loss of MMP-2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth. Hum. Mol. Genet. 2007, 16, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Matsuda, H.; Tanioka, M.; Kuwabara, K.; Itohara, S.; Suzuki, R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J. Immunol. 2002, 169, 2643–2647. [Google Scholar] [CrossRef] [PubMed]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Takagi, S.; Okumura, M.; Fujinaga, T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol. Bioeng. 2006, 93, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.H.; Kim, J.S.; Ahn, J.C.; Kim, H.C.; Kim, B.S.; Han, D.K.; Park, S.G.; Lee, S.H. The role of tauroursodeoxycholic acid on adipogenesis of human adipose-derived stem cells by modulation of ER stress. Biomaterials 2014, 35, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, L.; Sartori, M.F.; Albisetti, W.; Brini, A.T. Osteogenic differentiation of human adipose-derived stem cells: Comparison of two different inductive media. J. Tissue Eng. Regen. Med. 2007, 1, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa Ki, K.; Katayama, M.; Matsuda, Y.; Takahashi, H.; Hara, I.; Sato, H.; Kaneko, S. Matrix metalloproteinase (MMP)-2 and MMP-9 activities in human seminal plasma. Mol. Hum. Reprod. 2002, 8, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Seo, Y.K.; Yoon, H.H.; Song, K.Y.; Kwon, S.Y.; Lee, H.S.; Park, J.K. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 2008, 105, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, X.; Lin, S.; Li, X.; Zhang, S.; Song, Y.H. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007, 356, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Griffith, L.G.; Wells, A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res. Ther. 2010, 1, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, G.; Schmal, O.; Aicher, W.K. Matrix metalloproteinases in stem cell mobilization. Matrix Biol. 2015, 44–46, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Schwarzbauer, J.E. Fibronectin and stem cell differentiation—Lessons from chondrogenesis. J. Cell Sci. 2012, 125, 3703–3712. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, B.-S. Control of adult stem cell behavior with biomaterials. J. Tissue Eng. Regen. Med. 2014, 11, 423–430. [Google Scholar] [CrossRef]
- Cha, K.J.; Park, K.S.; Kang, S.W.; Cha, B.H.; Lee, B.K.; Han, I.B.; Shin, D.A.; Kim, D.S.; Lee, S.H. Effect of replicated polymeric substrate with lotus surface structure on adipose-derived stem cell behaviors. Macromol. Biosci. 2011, 11, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Pei, M. Extracellular matrix enhances differentiation of adipose stem cells from infrapatellar fat pad toward chondrogenesis. J. Tissue Eng. Regen. Med. 2013, 7, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.H.; Lai, W.F.; Chang, S.F.; Wong, C.C.; Fan, C.Y.; Fang, C.L.; Tsai, Y.H. The effect of type II collagen on MSC osteogenic differentiation and bone defect repair. Biomaterials 2014, 35, 2680–2691. [Google Scholar] [CrossRef] [PubMed]
- Salasznyk, R.M.; Klees, R.F.; Hughlock, M.K.; Plopper, G.E. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Commun. Adhes. 2004, 11, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Kuboki, Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J. Biochem. 2001, 129, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Rowe, R.G.; Botvinick, E.L.; Kurup, A.; Putnam, A.J.; Seiki, M.; Weaver, V.M.; Keller, E.T.; Goldstein, S.; Dai, J.; et al. MT1-MMP-dependent control of skeletal stem cell commitment via a β1-integrin/YAP/TAZ signaling axis. Dev. Cell 2013, 25, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Shukunami, C.; Morioka, Y.; Matsumoto, N.; Takahashi, R.; Oh, J.; Atsumi, T.; Umezawa, A.; Kudo, A.; Kitayama, H.; et al. Dual effects of the membrane-anchored MMP regulator RECK on chondrogenic differentiation of ATDC5 cells. J. Cell Sci. 2007, 120, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Lee, M.T.; Ke, F.C.; Lee, P.P.; Huang, C.J.; Ip, M.M.; Chen, L.; Hwang, J.J. TGFβ1 stimulates the secretion of matrix metalloproteinase 2 (MMP2) and the invasive behavior in human ovarian cancer cells, which is suppressed by MMP inhibitor BB3103. Clin. Exp. Metastasis 2000, 18, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Gepstein, A.; Shapiro, S.; Arbel, G.; Lahat, N.; Livne, E. Expression of matrix metalloproteinases in articular cartilage of temporomandibular and knee joints of mice during growth, maturation, and aging. Arthritis Rheum. 2002, 46, 3240–3250. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.J.; Choi, Y.A.; Kyun Park, E.; Bang, O.S.; Kang, S.S. MMP-2 functions as a negative regulator of chondrogenic cell condensation via down-regulation of the FAK-integrin beta1 interaction. Dev. Biol. 2007, 308, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Bhoopathi, P.; Chetty, C.; Gogineni, V.R.; Gujrati, M.; Dinh, D.H.; Rao, J.S.; Lakka, S.S. MMP-2 mediates mesenchymal stem cell tropism towards medulloblastoma tumors. Gene Ther. 2011, 18, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Estes, B.T.; Diekman, B.O.; Gimble, J.M.; Guilak, F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat. Protoc. 2010, 5, 1294–1311. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, E.; Niebur, G.L.; McHugh, P.E.; Shaw, G.; Barry, F.P.; McNamara, L.M. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cell. Mater. 2012, 23, 13–27. [Google Scholar] [PubMed]
- Kylmaniemi, M.; Oikarinen, A.; Oikarinen, K.; Salo, T. Effects of dexamethasone and cell proliferation on the expression of matrix metalloproteinases in human mucosal normal and malignant cells. J. Dent. Res. 1996, 75, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Peperzak, V.; van Rijn, L.; Borst, J.; de Bruijn, J.D. Dexamethasone treatment during the expansion phase maintains stemness of bone marrow mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2010, 4, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Medrado, G.C.; Machado, C.B.; Valerio, P.; Sanches, M.D.; Goes, A.M. The effect of a chitosan-gelatin matrix and dexamethasone on the behavior of rabbit mesenchymal stem cells. Biomed. Mater. 2006, 1, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Tsai, Y.H.; Deng, W.P.; Shih, S.N.; Fang, C.L.; Burch, J.G.; Chen, W.H.; Lai, W.F. Type I and II collagen regulation of chondrogenic differentiation by mesenchymal progenitor cells. J. Orthop. Res. 2005, 23, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Shintani, N.; Hunziker, E.B. Chondrogenic differentiation of bovine synovium: Bone morphogenetic proteins 2 and 7 and transforming growth factor beta1 induce the formation of different types of cartilaginous tissue. Arthritis Rheum. 2007, 56, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.B.; Fischer, M.; Zellner, J.; Berner, A.; Dienstknecht, T.; Prantl, L.; Kujat, R.; Nerlich, M.; Tuan, R.S.; Angele, P. Hypertrophy in mesenchymal stem cell chondrogenesis: Effect of TGF-β isoforms and chondrogenic conditioning. Cells Tissues Organs 2010, 192, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Cha, B.H.; Kim, J.S.; Ahn, J.; Han, I.; Park, H.; Lee, S.H. Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthr. Cartil. 2016, 24, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Leyh, M.; Seitz, A.; Durselen, L.; Schaumburger, J.; Ignatius, A.; Grifka, J.; Grassel, S. Subchondral bone influences chondrogenic differentiation and collagen production of human bone marrow-derived mesenchymal stem cells and articular chondrocytes. Arthritis Res. Ther. 2014, 16, 453. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.J.; Gilissen, C.; Roelofs, H.; Schaap-Oziemlak, A.; Veltman, J.A.; Raymakers, R.A.; Jansen, J.H.; Kogler, G.; Figdor, C.G.; Torensma, R.; et al. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 2010, 19, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl. Med. 2014, 12, 8. [Google Scholar] [CrossRef] [PubMed]







| Gene | Human Primer Sequence | |
|---|---|---|
| PPARγ | Sense | 5′-GATACACTGTCTGCAAACATATCACAA-3′ |
| Antisense | 5′-CCACGGAGCTGATCCCAA-3′ | |
| C/EBPβ | Sense | 5′-GCAAGAGCCGCGACAAG-3′ |
| Antisense | 5′-GGCTCGGGCAGCTGCTT-3′ | |
| COL II | Sense | 5′-CACGTACACTGCCCTGAAGGA-3′ |
| Antisense | 5′-CGATAACAGTCTTGCCCCACTT-3′ | |
| SOX9 | Sense | 5′-CCCCAACAGATCGCCTACAG-3′ |
| Antisense | 5′-GAGTTCTGGTCGGTGTAGTC-3′ | |
| RUNX2 | Sense | 5′-CAGACCAGCAGCACTCCATA-3′ |
| Antisense | 5′-CAGCGTCAACACCATCATTC-3′ | |
| COL I | Sense | 5′-CCCCTGGAAAGAATGGAGATG-3′ |
| Antisense | 5′-TCCAAACCACTGAAACCTCTG-3′ | |
| COL X | Sense | 5′-ACGCTGAACGATACCAAATG-3′ |
| Antisense | 5′-TGCTATACCTTTACTCTTTATGGTGTA-3′ | |
| MMP-13 | Sense | 5′-AACGCCAGACAAATGTGACC-3′ |
| Antisense | 5′-AGGTCATGAGAAGGGTGCTC-3′ | |
| RPS18 | Sense | 5′-CTTCCACAGGAGGCCTACAC-3′ |
| Antisense | 5′-CGCAAAATATGCTGGAACTTT-3′ | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, Y.; Park, S.; Choi, B.; Ko, K.-W.; Choi, W.C.; Lee, J.-M.; Han, D.-W.; Park, H.-K.; Han, I.; Lee, J.H.; et al. Enhancement of Matrix Metalloproteinase-2 (MMP-2) as a Potential Chondrogenic Marker during Chondrogenic Differentiation of Human Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2016, 17, 963. https://doi.org/10.3390/ijms17060963
Arai Y, Park S, Choi B, Ko K-W, Choi WC, Lee J-M, Han D-W, Park H-K, Han I, Lee JH, et al. Enhancement of Matrix Metalloproteinase-2 (MMP-2) as a Potential Chondrogenic Marker during Chondrogenic Differentiation of Human Adipose-Derived Stem Cells. International Journal of Molecular Sciences. 2016; 17(6):963. https://doi.org/10.3390/ijms17060963
Chicago/Turabian StyleArai, Yoshie, Sunghyun Park, Bogyu Choi, Kyoung-Won Ko, Won Chul Choi, Joong-Myung Lee, Dong-Wook Han, Hun-Kuk Park, Inbo Han, Jong Hun Lee, and et al. 2016. "Enhancement of Matrix Metalloproteinase-2 (MMP-2) as a Potential Chondrogenic Marker during Chondrogenic Differentiation of Human Adipose-Derived Stem Cells" International Journal of Molecular Sciences 17, no. 6: 963. https://doi.org/10.3390/ijms17060963
APA StyleArai, Y., Park, S., Choi, B., Ko, K. -W., Choi, W. C., Lee, J. -M., Han, D. -W., Park, H. -K., Han, I., Lee, J. H., & Lee, S. -H. (2016). Enhancement of Matrix Metalloproteinase-2 (MMP-2) as a Potential Chondrogenic Marker during Chondrogenic Differentiation of Human Adipose-Derived Stem Cells. International Journal of Molecular Sciences, 17(6), 963. https://doi.org/10.3390/ijms17060963