Melanoma-Derived BRAFV600E Mutation in Peritumoral Stromal Cells: Implications for in Vivo Cell Fusion
Abstract
:1. Introduction
2. Results
2.1. The BRAFV600E Protein Is Expressed in Subpopulations of Peritumoral Stromal Cells
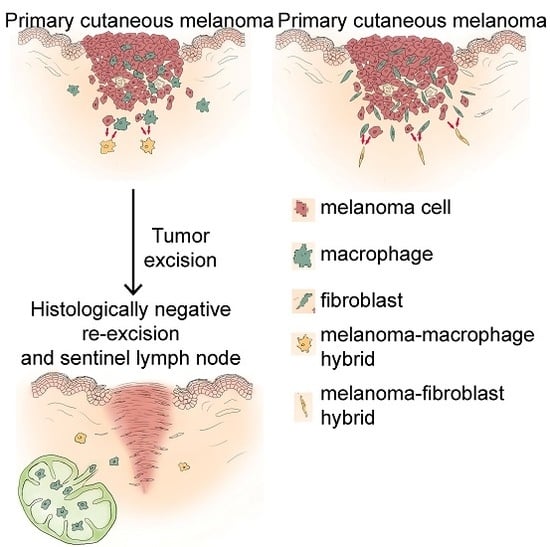
2.2. Some Peritumoral Fibroblasts and Macrophages Carry the BRAFV600E Mutation in Primary Melanoma, Melanoma Metastasis and a Tumor-Free Re-Excision Sample
3. Discussion
4. Experimental Section
4.1. Tissue Samples and Determination of BRAF Mutational Status
4.2. Immunohistochemistry
4.3. Laser-Capture Microdissection and Detection of the BRAFV600E Allele
4.4. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MART1 | melanoma antigen recognized by T-cells |
| SMA | smooth muscle actin |
References
- Hocevar, M.; Dragonja, Z.; Pilko, G.; Gazic, B.; Zgajnar, J. Residual melanoma after an excisional biopsy is an independent prognostic factor for local recurrence and overall survival. Eur. J. Surg. Oncol. 2014, 40, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.; Newton-Bishop, J.; A’Hern, R.; Coombes, G.; Timmons, M.; Evans, J.; Cook, M.; Theaker, J.; Fallowfield, M.; O’Neill, T.; et al. Excision margins in high-risk malignant melanoma. N. Engl. J. Med. 2004, 350, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Weinberg, R.A. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, J.; Kohlmeyer, J.; Renn, M.; Bald, T.; Rogava, M.; Cron, M.; Fatho, M.; Lennerz, V.; Wölfel, T.; Hölzel, M.; et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 2012, 490, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G. Mechanisms governing metastatic dormancy and reactivation. Cell 2013, 155, 750–764. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Easwaran, H.; Tsai, H.-C.; Baylin, S.B. Cancer epigenetics: Tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol. Cell 2014, 54, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Stoecklein, N.H.; Klein, C.A. Genetic disparity between primary tumours, disseminated tumour cells, and manifest metastasis. Int. J. Cancer 2010, 126, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Harkness, T.; Weaver, B.A.; Alexander, C.M.; Ogle, B.M. Cell fusion in tumor development: Accelerated genetic evolution. Crit. Rev. Oncog. 2013, 18, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Clawson, G.A. Cancer. Fusion for moving. Science 2013, 342, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Duelli, D.; Lazebnik, Y. Cell fusion: A hidden enemy? Cancer Cell 2003, 3, 445–448. [Google Scholar] [CrossRef]
- Rappa, G.; Mercapide, J.; Lorico, A. Spontaneous formation of tumorigenic hybrids between breast cancer and multipotent stromal cells is a source of tumor heterogeneity. Am. J. Pathol. 2012, 180, 2504–2515. [Google Scholar] [CrossRef] [PubMed]
- Shabo, I.; Midtbö, K.; Andersson, H.; Åkerlund, E.; Olsson, H.; Wegman, P.; Gunnarsson, C.; Lindström, A. Macrophage traits in cancer cells are induced by macrophage-cancer cell fusion and cannot be explained by cellular interaction. BMC Cancer 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Terada, N.; Hamazaki, T.; Oka, M.; Hoki, M.; Mastalerz, D.M.; Nakano, Y.; Meyer, E.M.; Morel, L.; Petersen, B.E.; Scott, E.W. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002, 416, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Kemény, L.V.; Kurgyis, Z.; Buknicz, T.; Groma, G.; Jakab, Á.; Zänker, K.; Dittmar, T.; Kemény, L.; Németh, I.B. Melanoma cells can adopt the phenotype of stromal fibroblasts and macrophages by spontaneous cell fusion in vitro. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Clawson, G.A.; Matters, G.L.; Xin, P.; Imamura-Kawasawa, Y.; Du, Z.; Thiboutot, D.M.; Helm, K.F.; Neves, R.I.; Abraham, T. Macrophage-tumor cell fusions from peripheral blood of melanoma patients. PLoS ONE 2015, 10, e0134320. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhu, Y.; Sun, Z.; Ji, R.; Zhang, X.; Xu, W.; Yuan, X.; Zhang, B.; Yan, Y.; Yin, L.; et al. Tumorigenic hybrids between mesenchymal stem cells and gastric cancer cells enhanced cancer proliferation, migration and stemness. BMC Cancer 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, B.; Shao, Y.; Zhao, N.; Hsu, Y.; Zhang, Z.; Zhu, L. Cell fusion between gastric epithelial cells and mesenchymal stem cells results in epithelial-to-mesenchymal transition and malignant transformation. BMC Cancer 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Hoon, D.S.B.; Pilati, P.; Rossi, C.R.; Nitti, D. Sentinel lymph node molecular ultrastaging in patients with melanoma: A systematic review and meta-analysis of prognosis. J. Clin. Oncol. 2007, 25, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Itakura, E.; Huang, R.-R.; Wen, D.-R.; Cochran, A.J. “Stealth” melanoma cells in histology-negative sentinel lymph nodes. Am. J. Surg. Pathol. 2011, 35, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Satzger, I.; Völker, B.; Meier, A.; Schenck, F.; Kapp, A.; Gutzmer, R. Prognostic significance of isolated HMB45 or Melan A positive cells in Melanoma sentinel lymph nodes. Am. J. Surg. Pathol. 2007, 31, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; DeSilva, C.; McCarthy, S.W.; Thompson, J.F.; Scolyer, R.A. Sentinel lymph nodes containing very small (<0.1 mm) deposits of metastatic melanoma cannot be safely regarded as tumor-negative. Ann. Surg. Oncol. 2012, 19, 1089–1099. [Google Scholar] [PubMed]
- Murali, R.; Thompson, J.F.; Shaw, H.M.; Scolyer, R.A. The prognostic significance of isolated immunohistochemically positive cells in sentinel lymph nodes of melanoma patients. Am. J. Surg. Pathol. 2008, 32, 1106–1108. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, A.; Dietz, K.; Hodak, I.; Polzer, B.; Scheitler, S.; Yildiz, M.; Czyz, Z.; Lehnert, P.; Fehm, T.; Hafner, C.; et al. Quantitative measurement of melanoma spread in sentinel lymph nodes and survival. PLoS Med. 2014, 11, e1001604. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.K.; Jones, W.O.; Shaw, J.H. Analysis of local recurrence and optimizing excision margins for cutaneous melanoma. Br. J. Surg. 2001, 88, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.E.; Maithel, S.K.; Carlson, G.W.; Rizzo, M.; Murray, D.R.; Hestley, A.C.; Delman, K.A. 1 or 2 cm margins of excision for T2 melanomas: Do they impact recurrence or survival? Ann. Surg. Oncol. 2013, 20, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Haydu, L.E.; Scolyer, R.A.; Winstanley, J.B.; Spillane, A.J.; Quinn, M.J.; Saw, R.P.M.; Shannon, K.F.; Stretch, J.R.; Thompson, J.F. The importance of adequate primary tumor excision margins and sentinel node biopsy in achieving optimal locoregional control for patients with thick primary melanomas. Ann. Surg. 2013, 258, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.E.; Cohen, C.; Siddiqui, M.T.; Palma, J.F.; Lipford, E.H.; Longshore, J.W. Accurate detection of BRAF p.V600E mutations in challenging melanoma specimens requires stringent immunohistochemistry scoring criteria or sensitive molecular assays. Hum. Pathol. 2014, 45, 2281–2293. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Wilmott, J.S.; Capper, D.; Preusser, M.; Zhang, Y.E.; Thompson, J.F.; Kefford, R.F.; von Deimling, A.; Scolyer, R.A. Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am. J. Surg. Pathol. 2013, 37, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Rachkovsky, M.; Sodi, S.; Chakraborty, A.; Avissar, Y.; Bolognia, J.; McNiff, J.M.; Platt, J.; Bermudes, D.; Pawelek, J. Melanoma × macrophage hybrids with enhanced metastatic potential. Clin. Exp. Metastasis 1998, 16, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Nagler, C.; Hardt, C.; Zänker, K.S.; Dittmar, T. Co-cultivation of murine BMDCs with 67NR mouse mammary carcinoma cells give rise to highly drug resistant cells. Cancer Cell Int. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, X.; Wang, C.Y.; Hu, P.; Chu, C.-Y.; Liu, S.; Zhau, H.E.; Chung, L.W.K. Spontaneous cancer-stromal cell fusion as a mechanism of prostate cancer androgen-independent progression. PLoS ONE 2012, 7, e42653. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.K.; Sodi, S.; Rachkovsky, M.; Kolesnikova, N.; Platt, J.T.; Bolognia, J.L.; Pawelek, J.M. A Spontaneous murine melanoma lung metastasis comprised of host × tumor hybrids. Cancer Res. 2000, 60, 2512–2519. [Google Scholar] [PubMed]
- Lazova, R.; LaBerge, G.S.; Duvall, E.; Spoelstra, N.; Klump, V.; Sznol, M.; Cooper, D.; Spritz, R.A.; Chang, J.T.; Pawelek, J.M. A Melanoma brain metastasis with a donor-patient hybrid genome following bone marrow transplantation: First evidence for fusion in human cancer. PLoS ONE 2013, 8, e66731. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Lazova, R.; Qumsiyeh, M.; Cooper, D.; Pawelek, J. Donor Y chromosome in renal carcinoma cells of a female BMT recipient: visualization of putative BMT-tumor hybrids by FISH. Bone Marrow Transplant. 2005, 35, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Nygren, J.M.; Liuba, K.; Breitbach, M.; Stott, S.; Thorén, L.; Roell, W.; Geisen, C.; Sasse, P.; Kirik, D.; Björklund, A.; et al. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat. Cell Biol. 2008, 10, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Espejel, S.; Romero, R.; Alvarez-Buylla, A. Radiation damage increases Purkinje neuron heterokaryons in neonatal cerebellum. Ann. Neurol. 2009, 66, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.B.; Youssef, S.; Koleckar, K.; Holbrook, C.; Doyonnas, R.; Corbel, S.Y.; Steinman, L.; Rossi, F.M.V.; Blau, H.M. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat. Cell Biol. 2008, 10, 575–583. [Google Scholar] [CrossRef] [PubMed]


| Tissue 1 | Npos/Nex 2,3 | |
|---|---|---|
| Fibroblasts 4 Dissected | Macrophages 5 Dissected | |
| BRAFV600E primary melanoma | 2/2 | 1/1 |
| BRAFV600E melanoma metastasis | 2/4 | 2/4 |
| Histologically tumor-free tissue (from patients with BRAFV600E melanoma) | 0/4 | 1/3 |
| BRAFWT primary melanoma | 0/4 | 0/1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurgyis, Z.; Kemény, L.V.; Buknicz, T.; Groma, G.; Oláh, J.; Jakab, Á.; Polyánka, H.; Zänker, K.; Dittmar, T.; Kemény, L.; et al. Melanoma-Derived BRAFV600E Mutation in Peritumoral Stromal Cells: Implications for in Vivo Cell Fusion. Int. J. Mol. Sci. 2016, 17, 980. https://doi.org/10.3390/ijms17060980
Kurgyis Z, Kemény LV, Buknicz T, Groma G, Oláh J, Jakab Á, Polyánka H, Zänker K, Dittmar T, Kemény L, et al. Melanoma-Derived BRAFV600E Mutation in Peritumoral Stromal Cells: Implications for in Vivo Cell Fusion. International Journal of Molecular Sciences. 2016; 17(6):980. https://doi.org/10.3390/ijms17060980
Chicago/Turabian StyleKurgyis, Zsuzsanna, Lajos V. Kemény, Tünde Buknicz, Gergely Groma, Judit Oláh, Ádám Jakab, Hilda Polyánka, Kurt Zänker, Thomas Dittmar, Lajos Kemény, and et al. 2016. "Melanoma-Derived BRAFV600E Mutation in Peritumoral Stromal Cells: Implications for in Vivo Cell Fusion" International Journal of Molecular Sciences 17, no. 6: 980. https://doi.org/10.3390/ijms17060980
APA StyleKurgyis, Z., Kemény, L. V., Buknicz, T., Groma, G., Oláh, J., Jakab, Á., Polyánka, H., Zänker, K., Dittmar, T., Kemény, L., & Németh, I. B. (2016). Melanoma-Derived BRAFV600E Mutation in Peritumoral Stromal Cells: Implications for in Vivo Cell Fusion. International Journal of Molecular Sciences, 17(6), 980. https://doi.org/10.3390/ijms17060980