TSPO PIGA Ligands Promote Neurosteroidogenesis and Human Astrocyte Well-Being
Abstract
:1. Introduction
2. Results
2.1. N,N-Dialkyl-2-phenylindol-3-ylglyoxylamide (PIGA) Ligands Increase the Oxidative Metabolism Activity/Proliferation Index in a Human Astrocyte Model
2.2. PIGA Ligands Effectively Stimulate Steroidogenesis in Vitro
2.3. The Best Performing PIGA Ligands in Terms of Steroidogenesis Stimulation Are Characterized by a Long Residence Time
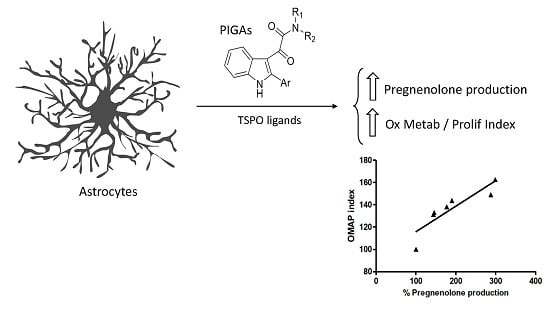
2.4. The Oxidative Metabolism Activity/Proliferation Index of PIGA1138 Is Related to Steroid Production
2.5. PIGA1138 Promoted the Activation of Oxidative Metabolism in Normal Human Astrocytes
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Drugs
4.3. Cell Culture
4.4. The Oxidative Metabolism Activity/Proliferation Index in the Astrocyte Cell Models
4.5. Pregnenolone Quantification
4.6. RT Determination of the TSPO PIGA Ligands
4.7. Radiolabel Binding Experiment in Human Astrocytes Using [3H] PK11195
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rupprecht, R.; Holsboer, F. Neuroactive steroids: Mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999, 9, 410–416. [Google Scholar] [CrossRef]
- Reddy, D.S. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog. Brain Res. 2010, 186, 113–137. [Google Scholar] [PubMed]
- Pfaff, D.W.; Gerlach, J.L.; McEwen, B.S.; Ferin, M.; Carmel, P.; Zimmerman, E.A. Autoradiographic localization of hormone-concentrating cells in the brain of the female rhesus monkey. J. Comp. Neurol. 1976, 170, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Buisson, B.; Bertrand, D. Steroid modulation of the nicotinic acetylcholine receptor. In Neurosteroids: A New Regulatory Function in the Nervous System, 1999th ed.; Baulieu, E.E., Robel, P., Schumacher, M., Eds.; Humana Press: Totowa, NJ, USA, 1999. [Google Scholar]
- Gibbs, T.T.; Yaghoubi, N.; Weaver, C.E., Jr.; Park-Chung, M.; Russek, S.J.; Farb, D.H. Modulation of ionotropic glutamate receptors by neuroactive steroids. In Neurosteroids: A New Regulatory Function in the Nervous System, 1999th ed.; Baulieu, E.E., Robel, P., Schumacher, M., Eds.; Humana Press: Totowa, NJ, USA, 1999; pp. 167–190. [Google Scholar]
- Majewska, M.D. Neurosteroid antagonists of the GABAA receptors. In Neurosteroids: A New Regulatory Function in the Nervous System, 1999th ed.; Baulieu, E.E., Robel, P., Schumacher, M., Eds.; Humana Press: Totowa, NJ, USA, 1999; pp. 155–166. [Google Scholar]
- Bastianetto, S.; Ramassamy, C.; Poirier, J.; Quirion, R. Dehydroepiandrosterone (DHEA) protects hippocampal cells from oxidative stress-induced damage. Mol. Brain Res. 1999, 6, 35–41. [Google Scholar] [CrossRef]
- Gee, K.W.; McCaule, L.D.; Lan, N.C. A putative receptor for neurosteroids on the GABAA receptor complex: The pharmacological properties and therapeutic potential of epalons. Crit. Rev. Neurobiol. 1995, 9, 207–227. [Google Scholar] [PubMed]
- Ueda, H.; Yoshida, A.; Tokuyama, S.; Mizuno, K.; Maruo, J.; Matsuno, K.; Mita, S. Neurosteroids stimulate G protein-coupled sigma receptors in mouse brain synaptic membrane. Neurosci. Res. 2001, 41, 33–40. [Google Scholar] [CrossRef]
- Schlichter, R.; Keller, A.F.; de Roo, M.; Breton, J.D.; Inquimbert, P.; Poisbeau, P. Fast nongenomic effects of steroids on synaptic transmission and role of endogenous neurosteroids in spinal pain pathways. J. Mol. Neurosci. 2006, 28, 33–51. [Google Scholar] [CrossRef]
- Herd, M.B.; Belelli, D.; Lambert, J.J. Neurosteroid modulation of synaptic and extrasynaptic GABA(A) receptors. Pharmacol. Ther. 2007, 116, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Magnaghi, V. GABA and neuroactive steroid interactions in glia: New roles for old players? Curr. Neuropharmacol. 2007, 5, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Duenas, M.; Luquin, S.; Chowen, J.A.; Torres-Aleman, I.; Naftolin, F.; Garcia-Segura, L.M. Gonadal hormone regulation of insulin-like growth factor-I-like immunoreactivity in hypothalamic astroglia of developing and adult rats. Neuroendocrinology 1994, 59, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, P.B.; Henshaw, R.; Weise, J.; Trubetskoy, V.; Finklestein, S.; Schulz, J.B.; Beal, M.F. Basic fibroblast growth factor protects against excitotoxicity and chemical hypoxia in both neonatal and adult rats. J. Cereb. Blood Flow Metab. 1995, 15, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.J.; Rozovsky, I.; Morgan, T.E.; Anderson, C.P.; Hajian, H.; Finch, C.E. Astrocytes and microglia respond to estrogen with increased apoE mRNA in vivo and in vitro. Exp. Neurol. 1997, 143, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.; Salmaso, N.; Cain, S.; Rodaros, D.; Stewart, J. Ovariectomy of adult rats leads to increased expression of astrocytic basic fibroblast growth factor in the ventral tegmental area and in dopaminergic projection regions of the entorhinal and prefrontal cortex. J. Neurosci. 1999, 19, 8665–8673. [Google Scholar] [PubMed]
- Buchanan, C.D.; Mahesh, V.B.; Brann, D.W. Estrogen-astrocyte-luteinizing hormone-releasing hormone signaling: A role for transforming growth factor-β1. Biol. Reprod. 2000, 62, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, M.; Martini, L.; Melcangi, R.C. Oestrogens, via transforming growth factor α, modulate basic fibroblast growth factor synthesis in hypothalamic astrocytes: In vitro observations. J. Neuroendocrinol. 2002, 14, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Kazanis, I.; Giannakopoulou, M.; Philippidis, H.; Stylianopoulou, F. Alterations in IGF-I, BDNF and NT-3 levels following experimental brain trauma and the effect of IGF-I administration. Exp. Neurol. 2004, 186, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Platania, P.; Seminara, G.; Aronica, E.; Troost, D.; Vincenza Catania, M.; Angela Sortino, M. 17β-estradiol rescues spinal motoneurons from AMPA-induced toxicity: A role for glial cells. Neurobiol. Dis. 2005, 20, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Mendez, P.; Cardona-Gomez, G.P.; Garcia-Segura, L.M. Interactions of insulin-like growth factor-I and estrogen in the brain. Adv. Exp. Med. Biol. 2005, 567, 285–303. [Google Scholar] [PubMed]
- Dhandapani, K.M.; Brann, D.W. Role of astrocytes in estrogen-mediated neuroprotection. Exp. Gerontol. 2007, 42, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Cerciat, M.; Unkila, M.; Garcia-Segura, L.M.; Arevalo, M.A. Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-gamma-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia 2010, 58, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.L.; Bi, C.W.; Choi, R.C.; Zhu, K.Y.; Miernisha, A.; Dong, T.T.; Tsim, K.W. Flavonoids induce the synthesis and secretion of neurotrophic factors in cultured rat astrocytes: A signaling response mediated by estrogen receptor. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.D.; Wisdom, A.J.; Cao, Y.; Hill, H.M.; Mongerson, C.R.; Stapornkul, B.; Itoh, N.; Sofroniew, M.V.; Voskuhl, R.R. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERα signaling on astrocytes but not through ERβ signaling on astrocytes or neurons. J. Neurosci. 2013, 33, 10924–10933. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Sidoryk-Wêgrzynowicz, M.; Wang, N.; Webb, A.; Son, D.S.; Lee, K.; Aschner, M. GPR30 regulates glutamate transporter GLT-1 expression in rat primary astrocytes. J. Biol. Chem. 2012, 287, 26817–26828. [Google Scholar] [CrossRef] [PubMed]
- Araújo, G.W.; Beyer, C.; Arnold, S. Oestrogen influences on mitochondrial gene expression and respiratory chain activity in cortical and mesencephalic astrocytes. J. Neuroendocrinol. 2008, 20, 930–941. [Google Scholar]
- Irwin, R.W.; Yao, J.; Hamilton, R.T.; Cadenas, E.; Brinton, R.D.; Nilsen, J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology 2008, 149, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Morohaku, K.; Pelton, S.H.; Daugherty, D.J.; Butler, W.R.; Deng, W.; Selvaraj, V. Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology 2014, 155, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.N.; Morohaku, K.; Manna, P.R.; Pelton, S.H.; Butler, W.R.; Stocco, D.M.; Selvaraj, V. Peripheral benzodiazepine receptor/translocator protein global knock-out mice are viable with no effects on steroid hormone biosynthesis. J. Biol. Chem. 2014, 289, 27444–27454. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Middleton, R.J.; Chan, R.; Hatty, C.R.; Kam, W.W.; Quin, C.; Graeber, M.B.; Parmar, A.; Zahra, D.; Callaghan, P.; et al. Positron emission tomography and functional characterization of a complete PBR/TSPO knockout. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Lecanu, L. Translocator protein (18 kDa) TSPO: An emerging therapeutic target in neurotrauma. Exp. Neurol. 2009, 219, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.A. Neurosteroids’ effects and mechanisms for social, cognitive, emotional, and physical functions. Psychoneuroendocrinology 2009, 34, S143–S161. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Campioli, E.; Midzak, A.; Culty, M.; Papadopoulos, V. Conditional steroidogenic cell-targeted deletion of TSPO Unveils a Crucial role in Viability and Hormone-Dependent Steroid Formation. Proc. Natl. Acad. Sci. USA 2015, 112, 7261–7266. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.H.; Deschepper, C.F. Neurosteroid biosynthesis: Genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993, 629, 283–292. [Google Scholar] [CrossRef]
- King, S.R.; Ginsberg, S.D.; Ishii, T.; Smith, R.G.; Parker, K.L.; Lamb, D.J. The steroidogenic acute regulatory protein is expressed in steroidogenic cells of the day-old brain. Endocrinology 2004, 145, 4775–4780. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Lacapère, J.J.; Papadopoulos, V. Peripheral-type benzodiazepine receptor: Structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids 2003, 68, 569–585. [Google Scholar] [CrossRef]
- Da Pozzo, E.; Costa, B.; Martini, C. Translocator protein (TSPO) and neurosteroids: Implications in psychiatric disorders. Curr. Mol. Med. 2012, 12, 426–442. [Google Scholar] [CrossRef] [PubMed]
- Da Pozzo, E.; Giacomelli, C.; Barresi, E.; Costa, B.; Taliani, S.; da Settimo, F.; Martini, C. Targeting the 18 kDa translocator protein: Recent perspectives for neuroprotection. Biochem. Soc. Trans. 2015, 43, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Veiga, S.; Azcoitia, I.; Garcia-Segura, L.M. Ro5-4864, a peripheral benzodiazepine receptor ligand, reduces reactive gliosis and protects hippocampal hilar neurons from kainic acid excitotoxicity. J. Neurosci. Res. 2005, 80, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Veiga, S.; Carrero, P.; Pernia, O.; Azcoitia, I.; Garcia-Segura, L.M. Translocator protein 18 kDa is involved in the regulation of reactive gliosis. Glia 2007, 55, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Girard, C.; Liu, S.; Cadepond, F.; Adams, D.; Lacroix, C.; Verleye, M.; Gillardin, J.M.; Baulieu, E.E.; Schumacher, M.; Schweizer-Groyer, G. Etifoxine improves peripheral nerve regeneration and functional recovery. Proc. Natl. Acad. Sci. USA 2008, 105, 20505–20510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Yu, J.Z.; Li, Q.Y.; Ma, C.G.; Lu, C.Z.; Xiao, B.G. TSPO-specific ligand vinpocetine exerts a neuroprotective effect by suppressing microglial inflammation. Neuron Glia Biol. 2011, 7, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Mattace Raso, G.; Taliani, S.; da Pozzo, E.; Simorini, F.; Costa, B.; Martini, C.; Laneri, S.; Sacchi, A.; Cosimelli, B.; et al. TSPO-ligands prevent oxidative damage and inflammatory response in C6 glioma cells by neurosteroid synthesis. Eur. J. Pharm. Sci. 2016, 88, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; da Pozzo, E.; Giacomelli, C.; Barresi, E.; Taliani, S.; da Settimo, F.; Martini, C. TSPO ligand residence time: A new parameter to predict compound neurosteroidogenic efficacy. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Tsou, T.C.; Chiu, I.M.; Chou, C.C. Proliferation inhibition, DNA damage, and cell-cycle arrest of human astrocytoma cells after acrylamide exposure. Chem. Res. Toxicol. 2010, 23, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, D.; Cheng, R.; Zhu, X.; Wan, T.; Liu, J.; Zhang, R. Mechanisms of U87 astrocytoma cell uptake and trafficking of monomeric versus protofibril Alzheimer’s disease amyloid-β proteins. PLoS ONE 2014, 9, e99939. [Google Scholar] [CrossRef] [PubMed]
- Maresca, B.; Spagnuolo, M.S.; Cigliano, L. Haptoglobin modulates β-amyloid uptake by U-87 MG astrocyte cell line. J. Mol. Neurosci. 2015, 56, 35–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz, L.; Kavanagh, M.E.; Phoa, A.F.; Heng, B.; Dzamko, N.; Chen, E.J.; Doddareddy, M.R.; Guillemin, G.J.; Kassiou, M. Optimisation of LRRK2 inhibitors and assessment of functional efficacy in cell-based models of neuroinflammation. Eur. J. Med. Chem. 2015, 95, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Da Settimo, F.; Simorini, F.; Taliani, S.; La Motta, C.; Marini, A.M.; Salerno, S.; Bellandi, M.; Novellino, E.; Greco, G.; Cosimelli, B.; et al. Anxiolytic-like effects of N,N-dialkyl-2-phenylindol-3-ylglyoxylamides by modulation of translocator protein promoting neurosteroid biosynthesis. J. Med. Chem. 2008, 51, 5798–5806. [Google Scholar] [CrossRef] [PubMed]
- Barresi, E.; Bruno, A.; Taliani, S.; Cosconati, S.; da Pozzo, E.; Salerno, S.; Simorini, F.; Daniele, S.; Giacomelli, C.; Marini, A.M.; et al. Deepening the topology of the Translocator Protein binding site by novel N,N-dialkyl-2-arylindol-3-ylglyoxylamides. J. Med. Chem. 2015, 58, 6081–6092. [Google Scholar] [CrossRef] [PubMed]
- Kugler, W.; Veenman, L.; Shandalov, Y.; Leschiner, S.; Spanier, I.; Lakomek, M.; Gavish, M. Ligands of the mitochondrial 18 kDa translocator protein attenuate apoptosis of human glioblastoma cells exposed to erucylphosphohomocholine. Cell Oncol. 2008, 30, 435–450. [Google Scholar] [PubMed]
- Banfalvi, G. Cell Cycle Synchronization. Methods and Protocols; Humana Press: Totowa, NJ, USA, 2011. [Google Scholar]
- Chen, M.; Huang, J.; Yang, X.; Liu, B.; Zhang, W.; Huang, L.; Deng, F.; Ma, J.; Bai, Y.; Lu, R.; Huang, B.; Gao, Q.; Zhuo, Y.; Ge, J. Serum starvation induced cell cycle synchronization facilitates human somatic cells reprogramming. PLoS ONE 2012, 7, e28203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, S.; Abe, T.; Gotoh, J.; Fukuuchi, Y. Substrate-dependence of reduction of MTT: A tetrazolium dye differs in cultured astroglia and neurons. Neurochem. Int. 2002, 40, 441–448. [Google Scholar] [CrossRef]
- Dunigan, D.D.; Waters, S.B.; Owen, T.C. Aqueous soluble tetrazolium/formazan MTS as an indicator of NADH- and NADPH-dependent dehydrogenase activity. Biotechniques 1995, 19, 640–649. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Guarneri, P.; Kreuger, K.E.; Guidotti, A.; Costa, E. Pregnenolone biosynthesis in C6-2B glioma cell mitochondria: Regulation by a mitochondrial diazepam binding inhibitor receptor. Proc. Natl. Acad. Sci. USA 1992, 89, 5113–5117. [Google Scholar] [CrossRef] [PubMed]
- Azcoitia, I.; Sierra, A.; Veiga, S.; Garcia-Segura, L.M. Aromatase expression by reactive astroglia is neuroprotective. Ann. N. Y. Acad. Sci. 2003, 1007, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, G.J.; van Muiswinkel, F.L.; Sturkenboom, J.M.; Wokke, J.H.; Bär, P.R.; van den Berg, L.H. Ovariectomy and 17β-estradiol modulate disease progression of a mouse model of ALS. Brain Res. 2004, 1021, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ovejero, D.; Azcoitia, I.; Doncarlos, L.L.; Melcangi, R.C.; Garcia-Segura, L.M. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res. Rev. 2005, 48, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Conejo, N.M.; González-Pardo, H.; Cimadevilla, J.M.; Argüelles, J.A.; Díaz, F.; Vallejo-Seco, G.; Arias, J.L. Influence of gonadal steroids on the glial fibrillary acidic protein-immunoreactive astrocyte population in young rat hippocampus. J. Neurosci. Res. 2005, 79, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.; Veiga, S.; Azcoitia, I.; Garcia-Segura, L.M.; Garcia-Ovejero, D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: Role of its metabolites, oestradiol and dihydrotestosterone. Eur. J. Neurosci. 2007, 25, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.I.; Lee, Y.D.; Gwag, B.J.; Cho, S.I.; Kim, S.S.; Suh-Kim, H. Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J. Neurol. Sci. 2008, 268, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, L.M. Hormones and Brain Plasticity; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Arevalo, M.A.; Santos-Galindo, M.; Bellini, M.J.; Azcoitia, I.; Garcia-Segura, L.M. Actions of estrogens on glial cells: Implications for neuroprotection. Biochim. Biophys. Acta 2010, 1800, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Gandolfo, P.; Patte, C.; Thoumas, J.L.; Leprince, J.; Vaudry, H.; Tonon, M.C. The endozepine ODN stimulates [3H]thymidine incorporation in cultured rat astrocytes. Neuropharmacology 1999, 38, 725–732. [Google Scholar] [CrossRef]
- Gandolfo, P.; Patte, C.; Leprince, J.; Régo, J.L.; Mensah-Nyagan, A.G.; Vaudry, H.; Tonon, M.C. The triakontatetraneuropeptide (TTN) stimulates thymidine incorporation in rat astrocytes through peripheral-type benzodiazepine receptors. J. Neurochem. 2000, 75, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Neary, J.T.; Jorgensen, S.L.; Oracion, A.M.; Bruce, J.H.; Norenberg, M.D. Inhibition of growth factor-induced DNA synthesis in astrocytes by ligands of peripheral-type benzodiazepine receptors. Brain Res. 1995, 675, 27–30. [Google Scholar] [CrossRef]
- Bruce, J.H.; Ramirez, A.M.; Lin, L.; Oracion, A.; Agarwal, R.P.; Norenberg, M.D. Peripheral-type benzodiazepines inhibit proliferation of astrocytes in culture. Brain Res. 1991, 564, 167–170. [Google Scholar] [CrossRef]
- Ikezaki, K.; Black, K.L. Stimulation of cell growth and DNA synthesis by peripheral benzodiazepine. Cancer Lett. 1990, 49, 115–120. [Google Scholar] [CrossRef]
- Miccoli, L.; Oudard, S.; Beurdeley-Thomas, A.; Dutrillaux, B.; Poupon, M.F. Effect of 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinoline carboxamide (PK11195), a specific ligand of the peripheral benzodiazepine receptor, on the lipid fluidity of mitochondria in human glioma cells. Biochem. Pharmacol. 1999, 58, 715–721. [Google Scholar] [CrossRef]
- Shiraishi, T.; Black, K.L.; Ikezaki, K.; Becker, D.P. Peripheral benzodiazepine induces morphological changes and proliferation of mitochondria in glioma cells. J. Neurosci. Res. 1991, 30, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Black, K.L.; Shiraishi, T.; Ikezak, K.; Tabuchi, K.; Becker, D.P. Peripheral benzodiazepine stimulates secretion of growth hormone and mitochondrial proliferation in pituitary tumour GH3 cells. Neurol. Res. 1994, 16, 74–80. [Google Scholar] [PubMed]
- Scarf, A.M.; Auman, K.M.; Kassiou, M. Is there any correlation between binding and functional effects at the translocator protein (TSPO) (18 kDa)? Curr. Mol. Med. 2012, 12, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Alho, H.; Varga, V.; Krueger, K.E. Expression of mitochondrial benzodiazepine receptor and its putative endogenous ligand diazepam binding inhibitor in cultured primary astrocytes and C-6 cells: Relation to cell growth. Cell Growth Differ. 1994, 5, 1005–1014. [Google Scholar] [PubMed]
- Gao, Z.W.; Huang, J.B.; Lin, Q.; Qin, Q.; Liang, Y.J.; Zhou, L.; Luo, M. The effects of PK11195 on meningioma was associated with allopregnanolone biosynthesis, which was mediated by translocator protein 18 kDa. Cancer Biomark. 2016, 16, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Hechter, O.; Halkerston, I.D. Effects of steroid hormones on gene regulation and cell metabolism. Annu. Rev. Physiol. 1965, 27, 133–162. [Google Scholar] [CrossRef] [PubMed]
- Takuma, K.; Baba, A.; Matsuda, T. Astrocyte apoptosis: Implications for neuroprotection. Prog. Neurobiol. 2004, 72, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.; Natarajan, A.; Grünaug, S.; Kroismayr, R.; Wagner, E.F.; Sibilia, M. Neuronal survival depends on EGFR signaling in cortical but not midbrain astrocytes. EMBO J. 2006, 25, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Elger, C.E.; Hans, V.H.; Schramm, J.; Urbach, H.; Lassmann, H.; Bien, C.G. Astrocytes are a specific immunological target in Rasmussen’s encephalitis. Ann. Neurol. 2007, 62, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Volpi, L.; Pasquali, L.; Petrozzi, L.; Siciliano, G. Astrocyte-neuron interactions in neurological disorders. J. Biol. Phys. 2009, 35, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M. Neuromyelitis optica spectrum disorders. Continuum (Minneap. Minn.) 2010, 16, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Rouach, N. Astroglial networking contributes to neurometabolic coupling. Front. Neuroenerg. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Phatnani, H.; Maniatis, T. Astrocytes in neurodegenerative disease. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef] [PubMed]





 | Compound | R1 | R2 | Ar | Ki (nM) a |
| PIGA1228 | (CH2)3CH3 | (CH2)3CH3 | 3-Thienyl | 2.83 ± 0.30 | |
| PIGA1248 | (CH2)5CH3 | (CH2)5CH3 | 3-Thienyl | 0.89 ± 0.10 | |
| PIGA1175 | (CH2)2CH3 | (CH2)2CH3 | p-Biphenyl | 0.53 ± 0.05 | |
| PIGA1165 | (CH2)3CH3 | (CH2)3CH3 | p-Biphenyl | 5.50 ± 1.00 | |
| PIGA1174 | (CH2)5CH3 | (CH2)5CH3 | p-Biphenyl | 1.84 ± 0.20 | |
| PIGA1128 | (CH2)2CH3 | (CH2)2CH3 | 2-Naphthyl | 0.30 ± 0.04 | |
| PIGA1136 | (CH2)3CH3 | (CH2)3CH3 | 2-Naphthyl | 0.53 ± 0.06 | |
| PIGA1130 | (CH2)5CH3 | (CH2)5CH3 | 2-Naphthyl | 0.52 ± 0.06 | |
| PIGA1137 | CH3 | (CH2)3CH3 | 2-Naphthyl | 0.56 ± 0.06 | |
| PIGA1138 | CH3 | (CH2)4CH3 | 2-Naphthyl | 0.37 ± 0.04 | |
| PIGA1226 | (CH2)3CH3 | (CH2)3CH3 | p-Methoxyphenyl | 20.3 ± 2.21 | |
| PIGA1244 | (CH2)5CH3 | (CH2)5CH3 | p-Methoxyphenyl | 4.04 ± 0.44 | |
| PIGA1212 a | (CH2)2CH3 | (CH2)2CH3 | p-Methylphenyl | 5.50 ± 0.38 | |
| PK 11195 b | 9.3 ± 0.50 |
| Compounds | Pregnenolone Production in C6 cells (% ± SEM) | p | Pregnenolone Production in U87MG cells (% ± SEM) | p |
|---|---|---|---|---|
| PIGA1128 | 148.4 ± 4.643 | <0.01 | 190.0 ± 8.356 | <0.001 |
| PIGA1136 | 164.0 ± 2.096 | <0.01 | 177.3 ± 7.963 | <0.001 |
| PIGA1130 | 155.0 ± 5.774 | <0.01 | 145.8 ± 3.393 | <0.01 |
| PIGA1137 | 208.0 ± 4.933 | <0.001 | 287.6 ± 11.98 | <0.001 |
| PIGA1138 | 245.0 ± 5.774 | <0.001 | 298.7 ± 10.14 | <0.001 |
| PK 11195 | 138.5 ± 9.717 | <0.05 | 143.8 ± 20.40 | <0.01 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Pozzo, E.; Giacomelli, C.; Costa, B.; Cavallini, C.; Taliani, S.; Barresi, E.; Da Settimo, F.; Martini, C. TSPO PIGA Ligands Promote Neurosteroidogenesis and Human Astrocyte Well-Being. Int. J. Mol. Sci. 2016, 17, 1028. https://doi.org/10.3390/ijms17071028
Da Pozzo E, Giacomelli C, Costa B, Cavallini C, Taliani S, Barresi E, Da Settimo F, Martini C. TSPO PIGA Ligands Promote Neurosteroidogenesis and Human Astrocyte Well-Being. International Journal of Molecular Sciences. 2016; 17(7):1028. https://doi.org/10.3390/ijms17071028
Chicago/Turabian StyleDa Pozzo, Eleonora, Chiara Giacomelli, Barbara Costa, Chiara Cavallini, Sabrina Taliani, Elisabetta Barresi, Federico Da Settimo, and Claudia Martini. 2016. "TSPO PIGA Ligands Promote Neurosteroidogenesis and Human Astrocyte Well-Being" International Journal of Molecular Sciences 17, no. 7: 1028. https://doi.org/10.3390/ijms17071028
APA StyleDa Pozzo, E., Giacomelli, C., Costa, B., Cavallini, C., Taliani, S., Barresi, E., Da Settimo, F., & Martini, C. (2016). TSPO PIGA Ligands Promote Neurosteroidogenesis and Human Astrocyte Well-Being. International Journal of Molecular Sciences, 17(7), 1028. https://doi.org/10.3390/ijms17071028