Effect of Medium Supplements on Agrobacterium rhizogenes Mediated Hairy Root Induction from the Callus Tissues of Camellia sinensis var. sinensis
Abstract
:1. Introduction
2. Results
2.1. Effect of Medium Supplements on Minimizing Callus Browning for Regular Subculture
2.2. Effect of Medium Supplements on Agrobacterium Growth Grown in Luria Bertani (LB) Broth
2.3. Effect of Medium Supplements on vir Gene Expression
2.4. Effect of Medium Supplements on Agrobacterium Growth and Tissue Browning in the Inoculation Study
2.4.1. Effects on Agrobacterium Growth
2.4.2. Effects on Callus Browning
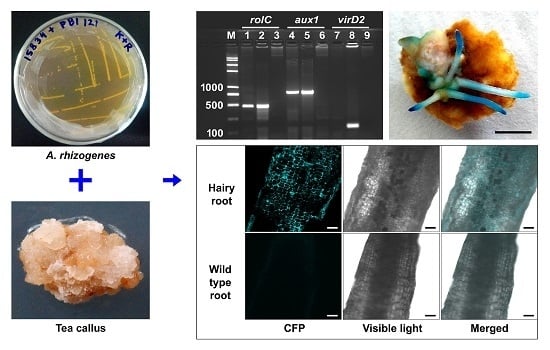
2.5. Effect of Medium Supplements on the Generation of Hairy Roots
3. Discussion
4. Materials and Methods
4.1. Explant Preparation and Callus Induction
4.2. Agrobacterium Strain and Transformation Vector
4.3. Medium Supplements
4.4. Experimental Design
4.5. Agrobacterium Growth and vir Gene Expression Assay Using LB Broth
4.6. Agrobacterium Inoculation to Callus and Induction of Hairy Roots
4.7. Data Collection
4.8. Analysis of the Hairy Roots Using PCR
4.9. β-Glucuronidase (GUS) and Cyan Fluorescent Protein (CFP) Expression in the Hairy Roots
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Experimental Parameters | Description |
|---|---|
| First experiment (Tissue culture study) | Completely randomized design |
| Treatments: MS media with different supplements | 9 (M1, M2, M3, M4, M5, M6, M7, M8, and M9) |
| Replications | 3 |
| Total experimental units | 27 (9 × 3 × 15 explants) |
| Second experiment (Agrobacterium growth and vir gene assay) | Completely randomized design |
| Treatments: LB broth media with different supplements | 9 (M1, M2, M3, M4, M5, M6, M7, M8, and M9) |
| Replications | 3 |
| Total experimental units | 27 (9 × 3) |
| Third experiment (Inoculation study) | Completely randomized design |
| Treatments: MS media with different supplements | 9 (M1, M2, M3, M4, M5, M6, M7, M8, and M9) |
| Replications | 6 |
| Total experimental units | 54 (9 × 6 × 15 explants) |
| Fourth experiment (Transformation study) | Completely randomized design |
| Treatments: MS media with different supplements | 5 (M1, M2, M3, M6, and M8) |
| Replications | 3 (50 explants in each) |
| Total experimental units | 15 (5 × 3 × 50 explants) |
References
- Mondal, T.K.; Bhattacharya, A.; Laxmikumaran, M.; Ahuja, P.S. Recent advances of tea (Camellia sinensis) biotechnology. Plant Cell Tissue Organ Cult. 2004, 76, 195–254. [Google Scholar] [CrossRef]
- De Costa, W.A.; Mohotti, A.J.; Wijeratne, M.A. Ecophysiology of tea. Braz. J. Plant Physiol. 2007, 19, 299–332. [Google Scholar] [CrossRef]
- Sandal, I.; Saini, U.; Lacroix, B.; Bhattacharya, A.; Ahuja, P.S.; Citovsky, V. Agrobacterium-mediated genetic transformation of tea leaf explants: Effects of counteracting bactericidity of leaf polyphenols without loss of bacterial virulence. Plant Cell Rep. 2007, 26, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gulati, A.; Bhattacharya, A. l-Glutamine and l-glutamic acid facilitate successful Agrobacterium infection of recalcitrant tea cultivars. Appl. Biochem. Biotechnol. 2013, 107, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Song, D.P.; Feng, L.; Rana, M.M.; Gao, M.J.; Wei, S. Effects of catechins on Agrobacterium mediated genetic transformation of Camellia sinensis. Plant Cell Tissue Organ Cult. 2014, 119, 27–37. [Google Scholar] [CrossRef]
- Ru, Z.; Lai, Y.; Xu, C.; Li, L. Polyphenol oxidase (PPO) in early stage of browning of Phalaenopsis leaf explants. J. Agric. Sci. 2013, 5, 57–64. [Google Scholar] [CrossRef]
- Lin, Y.S.; Tsai, Y.J.; Tsay, J.S.; Lin, J.K. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J. Agric. Food Chem. 2003, 51, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Murali, K.S.; Baby, U.I.; Manivel, L. Combating microbial contamination and browning in tea tissue culture. J. Plant. Crops 2001, 29, 55–58. [Google Scholar]
- Naz, S.; Ali, A.; Iqbal, J. Phenolic content in vitro cultures of chick pea (Cicer arietinum L.) during callogenesis and organogenesis. Pak. J. Bot. 2008, 40, 2525–2539. [Google Scholar]
- Ngomuo, M.; Mneney, E.; Ndakidemi, P. Control of lethal browning by using ascorbic acid on shoot tip cultures of a local Musa spp. (Banana) cv. Mzuzu in Tanzania. Afr. J. Biotechnol. 2014, 13, 1721–1725. [Google Scholar]
- Ahn, Y.J.; Kawamura, T.; Kim, M.; Yamamoto, T.; Mitsuoka, T. Tea polyphenols: Selective growth inhibitors of Clostridium spp. Agric. Biol. Chem. 1991, 55, 1425–1426. [Google Scholar] [CrossRef]
- Yam, T.S.; Shah, S.; Hamilton-Miller, J.M. Microbiological activity of whole and fractionated crude extracts of tea (Camellia sinensis), and of tea components. FEMS Microbiol. Lett. 1997, 152, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Mondal, T.K.; Bhattacharya, A.; Ahuja, P.S.; Chand, P.K. Transgenic tea (Camellia sinensis (L.) O. Kuntze cv. Kangra Jat) plants obtained by Agrobacterium-mediated transformation of somatic embryos. Plant Cell Rep. 2001, 20, 712–720. [Google Scholar]
- Lopez, S.J.; Kumar, R.R.; Pius, P.K.; Muraleedharan, N. Agrobacterium tumefaciens-mediated genetic transformation in tea (Camellia sinensis (L.) O. Kuntze). Plant Mol. Biol. Rep. 2004, 22, 201–202. [Google Scholar] [CrossRef]
- Ozawa, K. Establishment of a high efficiency Agrobacterium-mediated transformation system of rice (Oryza sativa L.). Plant Sci. 2009, 176, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Schrammeijer, B.; Beijersbergen, A.; Idler, K.B.; Melchers, L.S.; Thompson, D.V.; Hooykaas, P.J. Sequence analysis of the vir-region from Agrobacterium tumefaciens octopine Ti plasmid pTi15955. J. Exp. Bot. 2000, 51, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Gelvin, S.B. Agrobacterium and plant genes involved in T-DNA transfer and integration. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 223–256. [Google Scholar] [CrossRef] [PubMed]
- Toth, K.; Haapala, T.; Hohtola, A. Alleviation of browning in oak explants by chemical pretreatments. Biol. Plant 1994, 36, 511–517. [Google Scholar] [CrossRef]
- Feriz, M.K.; Zamani, Z.; Ebadi, A. In vitro establishment of three olive cultivars (Olea europaea L.) Gorgan. J. Agric. Sci. Nat. Resour. 2005, 12, 29–37. [Google Scholar]
- Bernal, M.P.; Hernández, C.; Barceló, M.T.; Delgado, M.; Armas, R. Quantitative transient GUS expression in J-104 rice calli through manipulation of in vitro culture conditions. Rev. Colomb. Biotecnol. 2009, 11, 75–84. [Google Scholar]
- Uchendu, E.E.; Paliyath, G.; Brown, D.C.W.; Saxena, P.K. In vitro propagation of the North American ginseng (Panax quinquefolius L.). In Vitro Cell. Dev. Biol. Plant 2011, 47, 710–718. [Google Scholar] [CrossRef]
- Jones, A.M.P.; Saxena, P.K. Inhibition of phenylpropanoid biosynthesis in Artemisia annua L.: A novel approach to reduce oxidative browning in plant tissue culture. PLoS ONE 2013, 8, e76802. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Bakshi, S.; Sahoo, D.P.; Kalita, M.C.; Sahoo, L. Agrobacterium-mediated genetic transformation of Pogostemon cablin (Blanco) Benth. Using leaf explants: Bactericidal effect of leaf extracts and counteracting strategies. Appl. Biochem. Biotechnol. 2012, 166, 1871–1895. [Google Scholar] [CrossRef] [PubMed]
- Stachel, S.E.; Messens, E.; van Montagu, M.; Zambryski, P. Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 1985, 318, 624–629. [Google Scholar] [CrossRef]
- Sheng, J.; Citovsky, V. Agrobacterium-plant cell DNA transport: Have virulence proteins, will travel. Plant Cell 1996, 8, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Gnasekaran, P.; Antony, J.J.J.; Uddain, J.; Subramaniam, S. Agrobacterium-mediated transformation of the recalcitrant Vanda Kasem’s Delight orchid with higher efficiency. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Jeyaramraja, P.R.; Meenakshi, S.N. Agrobacterium tumefaciens-mediated transformation of embryogenic tissues of tea (Camellia sinensis (L.) O. Kuntze). Plant Mol. Biol. Rep. 2005, 23, 299–300. [Google Scholar] [CrossRef]
- Sandal, I.; Koul, R.; Saini, U.; Mehta, M.; Dhiman, N.; Kumar, N.; Ahuja, P.S.; Bhattacharya, A. Development of transgenic tea plants from leaf explants by the biolistic gun method and their evaluation. Plant Cell Tissue Organ Cult. 2015, 123, 245–255. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Singh, H.R.; Agarwala, N.; Bhagawati, P.; Ahmed, G.; Das, S. Agrobacterium mediated transfer of nptll and gus genes in Camellia assamica. J. Agric. Biotechnol. Sustain. Dev. 2014, 6, 22–28. [Google Scholar]
- Jartoodeh, S.V.; Davarynejad, G.H.; Tehranifar, A.; Kaveh, H.; Bisheh, H.A. Reducing browning problem in micro propagation of three pear cultivars; Sebri, Shekari and Natanz. Curr. Opin. Agric. 2013, 2, 25–27. [Google Scholar]
- Daly, C.G. Anti-bacterial effect of citric acid treatment of periodontally diseased root surfaces in vitro. J. Clin. Periodontol. 1982, 9, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.S. Effect of LED light radiation on antioxidant activity and Agrobacterium-mediate transformation of azuki bean epicotyls. Int. J. Sci. Eng. 2013, 3, 23–32. [Google Scholar]
- Doner, L.W.; Becard, G.; Irwin, P.L. Binding of flavonoids by polyvinylpolypyrrolidone. J. Agric. Food Chem. 1993, 41, 753–757. [Google Scholar] [CrossRef]
- Laborde, B.; Moine-Ledoux, V.; Richard, T.; Saucier, C.; Dubourdieu, D.; Monti, J.P. PVPP-Polyphenol complexes: A molecular approach. J. Agric. Food Chem. 2006, 54, 4383–4389. [Google Scholar] [CrossRef] [PubMed]
- Nester, E.W. Agrobacterium: Nature’s genetic engineer. Front. Plant Sci. 2015, 5, 730. [Google Scholar] [CrossRef] [PubMed]
- Subramoni, S.; Nathoo, N.; Klimov, E.; Yuan, Z.C. Agrobacterium tumefaciens responses to plant-derived signaling molecules. Front. Plant Sci. 2014, 5, 322. [Google Scholar] [CrossRef] [PubMed]
- Fronzes, R.; Christie, P.J.; Waksman, G. The structural biology of type IV secretion systems. Nat. Rev. Microbiol. 2009, 7, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Uppalapati, S.R.; Ryu, C.M.; Allen, S.N.; Kang, L.; Tang, Y.; Mysore, K.S. Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens. Plant Physiol. 2008, 146, 703–715. [Google Scholar] [CrossRef] [PubMed]
- De la Riva, G.A.; Gonzalez-Cabrera, J.; Vazquez-Padron, R.; Ayra-Pardo, C. Agrobacterium tumefaciens: A natural tool for plant transformation. Electron. J. Biotechnol. 1998, 1, 118–133. [Google Scholar] [CrossRef]
- Pitzschke, A. Agrobacterium infection and plant defense-transformation success hangs by a thread. Front. Plant Sci. 2013, 4, 519. [Google Scholar] [CrossRef] [PubMed]
- Yanofsky, M.F.; Nester, E.W. Molecular characterization of a host-range-determining locus from Agrobacterium tumefaciens. J. Bacteriol. 1986, 168, 244–250. [Google Scholar] [PubMed]
- Regensburg-Tuink, A.J.G.; Hooykaas, P.J.J. Transgenic N. glauca plants expressing bacterial virulence gene virF are converted into hosts for nopaline strains of A. tumefaciens. Nature 1993, 363, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Huffman, G. Microprojectile bombardment of plant tissues increased transformation frequency of Agrobacterium tumefaciens. Plant Mol. Biol. 1992, 18, 301–313. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension culture of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Anand, A.; Krichevsky, A.; Schornack, S.; Lahaye, T.; Tzfira, T.; Tang, Y.; Citovsky, V.; Mysore, K.S. Arabidopsis VirE2 INTERACTING PROTEIN2 is required for Agrobacterium T-DNA integration in plants. Plant Cell 2007, 19, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Medina-Bolivar, F.; Condori, J.; Rimando, A.M.; Hubstenberger, J.; Shelton, K.; O’Keefe, S.F.; Bennett, S.; Dolan, M.C. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry 2007, 68, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [PubMed]
- Russell, O.F. MSTAT–C v. 2.1 (A Computer Based Data Analysis Software); Crop and Soil Science Department, Michigan State University: East Lansing, MI, USA, 1994. [Google Scholar]








| Medium | Supplements |
|---|---|
| M1 | MS salts + Sucrose (30 g·L−1) (control) |
| M2 | MS salts + Sucrose (5 g·L−1) + l-glutamine (0.1 g·L−1) |
| M3 | MS salts + Sucrose (30 g·L−1) + l-glutamine (0.1 g·L−1) |
| M4 | MS salts + Sucrose (30 g·L−1) + acetosyringone (150 μM) |
| M5 | MS salts + Sucrose (30 g·L−1) + l-glutamine (0.1 g·L−1) + DTT (0.15 g·L−1) |
| M6 | MS salts + Sucrose (30 g·L−1) + l-glutamine (0.1 g·L−1) + PVPP (5 g·L−1) |
| M7 | MS salts + Sucrose (30 g·L−1) + l-glutamine (0.1 g·L−1) + Citric Acid (0.1 g·L−1) |
| M8 | MS salts + Sucrose (30 g·L−1) + l-glutamine (0.1 g·L−1) + DTT (0.15 g·L−1) + PVPP (5 g·L−1) |
| M9 | MS salts + Sucrose (30 g·L−1) + l-glutamine (0.1 g·L−1) + DTT (0.15 g·L−1) + PVPP (5 g·L−1) + Citric Acid (0.1 g·L−1) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, M.M.; Han, Z.-X.; Song, D.-P.; Liu, G.-F.; Li, D.-X.; Wan, X.-C.; Karthikeyan, A.; Wei, S. Effect of Medium Supplements on Agrobacterium rhizogenes Mediated Hairy Root Induction from the Callus Tissues of Camellia sinensis var. sinensis. Int. J. Mol. Sci. 2016, 17, 1132. https://doi.org/10.3390/ijms17071132
Rana MM, Han Z-X, Song D-P, Liu G-F, Li D-X, Wan X-C, Karthikeyan A, Wei S. Effect of Medium Supplements on Agrobacterium rhizogenes Mediated Hairy Root Induction from the Callus Tissues of Camellia sinensis var. sinensis. International Journal of Molecular Sciences. 2016; 17(7):1132. https://doi.org/10.3390/ijms17071132
Chicago/Turabian StyleRana, Mohammad M., Zhuo-Xiao Han, Da-Peng Song, Guo-Feng Liu, Da-Xiang Li, Xiao-Chun Wan, Alagarsamy Karthikeyan, and Shu Wei. 2016. "Effect of Medium Supplements on Agrobacterium rhizogenes Mediated Hairy Root Induction from the Callus Tissues of Camellia sinensis var. sinensis" International Journal of Molecular Sciences 17, no. 7: 1132. https://doi.org/10.3390/ijms17071132
APA StyleRana, M. M., Han, Z. -X., Song, D. -P., Liu, G. -F., Li, D. -X., Wan, X. -C., Karthikeyan, A., & Wei, S. (2016). Effect of Medium Supplements on Agrobacterium rhizogenes Mediated Hairy Root Induction from the Callus Tissues of Camellia sinensis var. sinensis. International Journal of Molecular Sciences, 17(7), 1132. https://doi.org/10.3390/ijms17071132