Structural Analysis of Hand Drawn Bumblebee Bombus terrestris Silk
Abstract
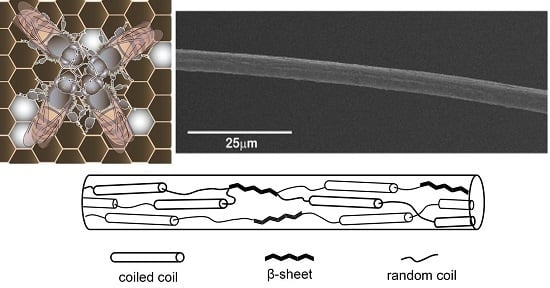
:1. Introduction
2. Results and Discussion
2.1. Microscopic Observations
2.2. Raman Spectra
2.3. Protein Conformation
2.4. Protein Orientation
3. Materials and Methods
3.1. Silk
3.2. Microscopic Observations
3.3. Raman Spectroscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sutherland, T.D.; Weisman, S.; Trueman, H.E.; Sriskantha, A.; Trueman, J.W.; Haritos, V.S. Conservation of essential design features in coiled coil silks. Mol. Biol. Evol. 2007, 24, 2424–2432. [Google Scholar] [CrossRef] [PubMed]
- Atkins, E.D.T. A four-strand coiled coil model for some insect fibrous proteins. J. Mol. Biol. 1967, 24, 139–141. [Google Scholar] [CrossRef]
- Crewe, R.M.; Thompson, P.R. Oecophylla silk: Functional adaptation in a biopolymer. Naturwissenschaften 1979, 66, 57–58. [Google Scholar] [CrossRef]
- Kameda, T.; Kojima, K.; Sezutsu, H.; Zhang, Q.; Teramoto, H.; Tamada, Y. Hornet (Vespa) Silk composed of coiled-coil proteins. Kobunshi Ronbunshu 2010, 67, 641–653. [Google Scholar] [CrossRef]
- Kameda, T.; Kojima, K.; Togawa, E.; Sezutsu, H.; Zhang, Q.; Teramoto, H.; Tamada, Y. Drawing-induced changes in morphology and mechanical properties of hornet silk gel films. Biomacromolecules 2010, 11, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.D.; Church, J.S.; Hu, X.; Huson, M.G.; Kaplan, D.L.; Weisman, S. Single honeybee silk protein mimics properties of multi-protein silk. PLoS ONE 2011, 6, e16489. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.D.; Weisman, S.; Walker, A.A.; Mudie, S.T. The coiled coil silk of bees, ants, and hornets. Biopolymers 2012, 97, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.O.H. Can alloethism in workers of the bumblebee, Bombus terrestris, be explained in terms of foraging efficiency? Anim. Behav. 2002, 64, 123–130. [Google Scholar]
- Widmer, A.; Schmid-Hempel, P.; Estoup, A.; Scholl, A. Population genetic structure and colonization history of Bombus terrestris s.l. (Hymenoptera: Apidae) from the Canary Islands and Madeira. Heredity 1998, 81, 563–572. [Google Scholar] [CrossRef]
- Inoue, M.K.; Yokoyama, J.; Washitani, I. Displacement of Japanese native bumblebees by the recently introduced Bombus terrestris (L.) (Hymenoptera: Apidae). J. Insect Conserv. 2008, 12, 135–146. [Google Scholar] [CrossRef]
- Torretta, J.A.; Medan, D.; Abrahamovich, A.H. First record of the invasive bumblebee Bombus terrestris (L.) (Hymenoptera, Apidae) in Argentina. Trans. Am. Entomol. Soc. (Phila.) 2006, 132, 285–289. [Google Scholar]
- Dornhaus, A.; Chittka, L. Food alert in bumblebees (Bombus terrestris): Possible mechanisms and evolutionary implications. Behav. Ecol. Sociobiol. 2001, 50, 570–576. [Google Scholar] [CrossRef]
- Church, J.S.; Woodhead, A.L.; Walker, A.A.; Sutherland, T.D. A comparison of convergently evolved insect silks that share β-sheet molecular structure. Biopolymers 2013, 101, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, T.; Paquet-Mercier, F.; Rioux-Dubé, J.-F.; Pézolet, M. Structure of silk by Raman spectromicroscopy: From the spinning glands to the fibers. Biopolymers 2012, 97, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, T.; Rousseau, M.-E.; Pézolet, M. Protein secondary structure and orientation in silk as revealed by Raman spectromicroscopy. Biophys. J. 2007, 92, 2885–2895. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, M.-E.; Lefèvre, T.; Beaulieu, L.; Asakura, T.; Pézolet, M. Study of protein conformation and orientation in silkworm and spider silk fibres using Raman microscopy. Biomacromolecules 2004, 5, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.; Church, J.S.; Woodhead, A.L.; Sutherland, T.D. Silverfish silk is formed by entanglement of randomly coiled protein chains. Insect Biochem. Mol. Biol. 2013, 43, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.A.; Weisman, S.; Church, J.S.; Merritt, D.J.; Mudie, S.T.; Sutherland, T.D. Silk from crickets: New twist on spinning. PLoS ONE 2012, 7, e30408. [Google Scholar] [CrossRef] [PubMed]
- Ishay, J.S.; Ganor, E. Comb cells and puparial silk in the oriental hornet nest: Structure and function. J. Morphol. 1990, 203, 11–19. [Google Scholar] [CrossRef]
- Siri, S.; Maensiri, S. Alternative biomaterials: Natural, non-woven, fibroin-based silk nanofibers of weaver ants (Oecophylla smaragdina). Int. J. Biol. Macromol. 2010, 46, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.G.M.; Farwell, D.W. Raman spectroscopic studies of silk. J. Raman Spectrosc. 1995, 26, 901–909. [Google Scholar] [CrossRef]
- Frushour, B.G.; Koenig, J.L. Raman spectroscopy of proteins. In Advances in Infrared and Raman Spectroscopy; Clark, R.S.H., Hester, R.E., Eds.; Heyden: New York, NY, USA, 1975; Volume 1, pp. 35–97. [Google Scholar]
- Monti, P.; Taddei, P.; Freddi, G.; Asakura, T.; Tsukada, M. Raman spectroscopic characterization of Bombyx mori silk fibroin: Raman spectrum of silk I. J. Raman Spectrosc. 2001, 32, 103–107. [Google Scholar] [CrossRef]
- Overman, S.A.; Thomas, G.J. Raman markers of nonaromatic side chains in an a-helix assembly: Ala, Asp, Glu, Gly, Ile, Leu, Lys, Ser, and Val residues of phage fd subunits. Biochemistry 1999, 38, 4018–4027. [Google Scholar] [CrossRef] [PubMed]
- Twardowshi, J.; Anzenbacher, P. Raman and IR Spectroscopy in Biology and Biochemistry; Ellis Horwood: New York, NY, USA, 1994. [Google Scholar]
- Sutherland, T.D.; Trueman, H.E.; Walker, A.A.; Weisman, S.; Campbell, P.M.; Dong, Z.; Huson, M.G.; Woodhead, A.L.; Church, J.S. Convergently-evolved structural anomalies in the coiled coil domains of insect silk proteins. J. Struct. Biol. 2014, 186, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.D.; Sriskantha, A.; Church, J.S.; Strive, T.; Trueman, H.; Kameda, T. Stabilization of viruses by encapsulation in silk proteins. ACS Appl. Mater. Interfaces 2014, 6, 18189–18196. [Google Scholar] [CrossRef] [PubMed]
- Fisk, S.; Ikeda, R.M.; Chase, D.B.; Rabolt, J.F. Rotational invariants for polarized Raman spectroscopy. Appl. Spectrosc. 2003, 57, 1053–1057. [Google Scholar] [CrossRef]
- Lefèvre, T.; Rousseau, M.-E.; Paquet-Mercier, F. Orientation insensitive spectra for Raman microspectroscopy. Appl. Spectrosc. 2006, 60, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.; Church, J.S.; Woodhead, A.L.; Huson, M.; Sriskantha, A.; Kyratzis, I.L.; Sutherland, T.D. Continuous production of flexible fibers from transgenically-produced honeybee silk proteins. Macromol. Biosci. 2013, 13, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Geddes, A.J.; Parker, K.D.; Atkins, E.D.T.; Beighton, E. “Cross-b” conformation in proteins. J. Mol. Biol. 1968, 32, 343–358. [Google Scholar] [CrossRef]
- Bauer, F.; Bertinetti, L.; Masic, A.; Scheibel, T. Dependence of mechanical properties of lacewing egg stalks on relative humidity. Biomacromolecules 2012, 13, 3730–3735. [Google Scholar] [CrossRef] [PubMed]
- Bower, D.I. Investigation of molecular orientation distributions by polarized Raman scattering and polarized fluorescence. J. Polym. Sci. B Polym. Phys. 1972, 10, 2135–2153. [Google Scholar] [CrossRef]
- Jen, S.; Clark, N.A.; Pershan, P.S.; Priestley, E.B. Polarized raman scattering studies of orientational order in uniaxial liquid crystalline phases. J. Chem. Phys. 1977, 66, 4635–4661. [Google Scholar] [CrossRef]
- Turrell, G. Analysis of polarization measurements in raman microspectroscopy. J. Raman Spectrosc. 1984, 15, 103–108. [Google Scholar] [CrossRef]
- Turrell, G. Raman sampling. In Practical Raman Spectroscopy; Gardiner, D.J., Graves, P.R., Eds.; Springer-Verlag: Berlin, Germany, 1989; pp. 13–54. [Google Scholar]
- Church, J.S.; Poole, A.J.; Woodhead, A.L. The Raman analysis of films cast from dissolved feather keratin. Vib. Spectrosc. 2010, 53, 107–111. [Google Scholar] [CrossRef]
- Berne, B.J.; Pechukas, P.; Harp, G.D. Molecular reorientation in liquids and gases. J. Chem. Phys. 1968, 49, 3125–3129. [Google Scholar] [CrossRef]
- Pottel, H.; Herreman, W.; van der Meer, B.W.; Ameloot, M. On the significance of the fourth-rank orientational order parameter of fluorophores in membranes. Chem. Phys. 1986, 102, 37–44. [Google Scholar] [CrossRef]
- Tsuboi, M.; Ikeda, T.; Ueda, T. Raman microscopy of a small uniaxial crystal: Tetragonal aspartame. J. Raman Spectrosc. 1991, 22, 619–626. [Google Scholar] [CrossRef]
- Lagugne Labarthet, F.; Buffeteau, T.; Sourisseau, C. Analyses of the diffraction efficiencies, birefringence, and surface relief gratings on azobenzene-containing polymer films. J. Phys. Chem. B 1998, 102, 2654–2662. [Google Scholar] [CrossRef]
- Park, M.S.; Wong, Y.S.; Park, J.O.; Venkatraman, S.S.; Srinivasarao, M. A simple method for obtaining the information of orientation distribution using polarized Raman spectroscopy: Orientation study of structural units in poly(lactic acid). Macromolecules 2013, 44, 2120–2131. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]







| Frequency (cm−1) | Relative Intensity 1 | Tentative Assignments 1 |
|---|---|---|
| 1673 | w, sh | Amide I, β-sheet |
| 1651 | s | Amide I, coiled coil |
| 1605 | vw | (C=C) aromatic ring in F |
| 1576 | vvw | (C=C) aromatic ring in F and W |
| 1554 | vw | (C=C) aromatic ring in W |
| 1457 | m | δ(CH2) and δ(CH3) in poly(A) and poly(AG) |
| 1333 | w, sh | δ(CH) |
| 1309 | m | Amide III, coiled coil |
| 1277 | vw, sh | Amide III, random coil |
| 1163 | w | (C–C) |
| 1123 | w | (C–C) and ν(C–N) |
| 1106 | w | (C–C) and poly(A) (Cα–Cβ and ρCβH3) |
| 1084 | vw | (C–C) skeletal random coil, β-sheet |
| 1046 | m | (C–C) skeletal |
| 1006 | vw | (C=C) aromatic ring breathing in F and W |
| 981 | vw | ρ(CH3) |
| 908 | m | (C–C) skeletal, poly(A), coiled coil |
| 758 | w | ρ(CH3) and skeletal bend in poly(A) |
| 715 | w | (C–S) M, trans |
| 645 | vw, br | (C–S) M |
| 544 | vw, sh | Skeletal bending |
| 527 | w | Skeletal bending in poly(A) |
| 457 | vw | Skeletal bending |
| 415 | vw | Skeletal bending |
| 378 | vw | Skeletal bending |
| 229 | w | Skeletal bending |
| Band Position (cm−1) | Height (Counts) | Width (cm−1) | % Lorentzian | % Area | Assignment | |
|---|---|---|---|---|---|---|
| 2nd Derivative | Curve Fit | |||||
| 1692 | 1691 | 722 | 21 | 99 | 4 | β-turn |
| 1673 | 1675 | 1766 | 28 | 32 | 10 | β-sheet |
| 1652 | 1651 | 12,326 | 23 | 100 | 73 | coiled coil |
| 1633 | 1633 | 2222 | 30 | 38 | 14 | random coil |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodhead, A.L.; Sutherland, T.D.; Church, J.S. Structural Analysis of Hand Drawn Bumblebee Bombus terrestris Silk. Int. J. Mol. Sci. 2016, 17, 1170. https://doi.org/10.3390/ijms17071170
Woodhead AL, Sutherland TD, Church JS. Structural Analysis of Hand Drawn Bumblebee Bombus terrestris Silk. International Journal of Molecular Sciences. 2016; 17(7):1170. https://doi.org/10.3390/ijms17071170
Chicago/Turabian StyleWoodhead, Andrea L., Tara D. Sutherland, and Jeffrey S. Church. 2016. "Structural Analysis of Hand Drawn Bumblebee Bombus terrestris Silk" International Journal of Molecular Sciences 17, no. 7: 1170. https://doi.org/10.3390/ijms17071170
APA StyleWoodhead, A. L., Sutherland, T. D., & Church, J. S. (2016). Structural Analysis of Hand Drawn Bumblebee Bombus terrestris Silk. International Journal of Molecular Sciences, 17(7), 1170. https://doi.org/10.3390/ijms17071170