VEGF and FGF2 Improve Revascularization, Survival, and Oocyte Quality of Cryopreserved, Subcutaneously-Transplanted Mouse Ovarian Tissues
Abstract
:1. Introduction
2. Results
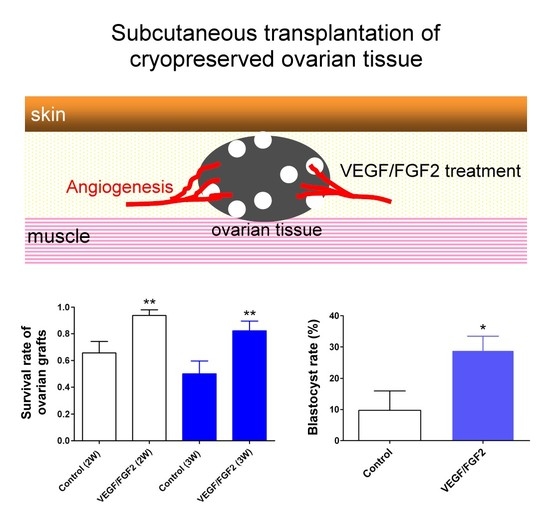
2.1. VEGF Coupled with FGF2 Improved Angiogenesis and Survival of Cryopreserved, Subcutaneously-Transplanted Mouse Ovarian Tissues
2.2. Levels of Angiogenic Cytokines in the Ovarian Grafts
2.3. VEGF and FGF2 Improved Oocyte Quality of the Transplanted Ovarian Tissues
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Vitrification of Ovarian Tissues
4.3. Treatment with VEGF and FGF2, and Autologous Subcutaneous Transplantation of the Vitrified-Thawed Ovarian Tissue
4.4. Graft Retrieval and Histological Analysis of Follicles
4.5. Immunohistochemical Staining of Vessels
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Gonadotropin Administration and Oocyte Retrieval
4.8. In Vitro Fertilization (IVF) and Embryo Culture
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix

References
- Practice Committee of the American Society for Reproductive Medicine; Practive Committee of the Society for Assisted Reproductive Technology. Ovarian tissue and oocyte cryopreservation. Fertil. Steril. 2006, 86, S142–S147. [Google Scholar]
- Poirot, C.J.; Martelli, H.; Genestie, C.; Golmard, J.L.; Valteau-Couanet, D.; Helardot, P.; Pacquement, H.; Sauvat, F.; Tabone, M.D.; Philippe-Chomette, P.; et al. Feasibility of ovarian tissue cryopreservation for prepubertal females with cancer. Pediatr. Blood Cancer 2007, 49, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Demeestere, I.; Simon, P.; Emiliani, S.; Delbaere, A.; Englert, Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum. Reprod. Update 2009, 15, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Jadoul, P.; Donnez, J.; Dolmans, M.M.; Squifflet, J.; Lengele, B.; Martinez-Madrid, B. Laparoscopic ovariectomy for whole human ovary cryopreservation: Technical aspects. Fertil. Steril. 2007, 87, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Madrid, B.; Camboni, A.; Dolmans, M.M.; Nottola, S.; van Langendonckt, A.; Donnez, J. Apoptosis and ultrastructural assessment after cryopreservation of whole human ovaries with their vascular pedicle. Fertil. Steril. 2007, 87, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Madrid, B.; Donnez, J. Cryopreservation of intact human ovary with its vascular pedicle—Or cryopreservation of hemiovaries? Hum. Reprod. 2007, 22, 1795–1796. [Google Scholar] [CrossRef] [PubMed]
- Shamonki, M.I.; Oktay, K. Oocyte and ovarian tissue cryopreservation: Indications, techniques, and applications. Semin. Reprod. Med. 2005, 23, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.M.; Demylle, D.; Jadoul, P.; Pirard, C.; Squifflet, J.; Martinez-Madrid, B.; van Langendonckt, A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004, 364, 1405–1410. [Google Scholar] [CrossRef]
- Donnez, J.; Squifflet, J.; Jadoul, P.; Demylle, D.; Cheron, A.C.; van Langendonckt, A.; Dolmans, M.M. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil. Steril. 2011, 95. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.; Bergholdt, S.; Jorgensen, J.S.; Andersen, C.Y. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum. Reprod. 2010, 25, 1280–1281. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.U.; Chien, C.L.; Wu, M.Y.; Chen, T.H.; Lai, S.M.; Lin, C.W.; Yang, Y.S. Novel direct cover vitrification for cryopreservation of ovarian tissues increases follicle viability and pregnancy capability in mice. Hum. Reprod. 2006, 21, 2794–2800. [Google Scholar] [CrossRef] [PubMed]
- Snow, M.; Cox, S.L.; Jenkin, G.; Trounson, A.; Shaw, J. Generation of live young from xenografted mouse ovaries. Science 2002, 297. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, T.; Cox, S.L.; Snow, M.; Jenkin, G.; Shaw, J. Offspring produced from heterotopic ovarian allografts in male and female recipient mice. Reproduction 2004, 127, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Yeoman, R.R.; Battaglia, D.E.; Stouffer, R.L.; Zelinski-Wooten, M.B.; Fanton, J.W.; Wolf, D.P. Live birth after ovarian tissue transplant. Nature 2004, 428, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Economos, K.; Kan, M.; Rucinski, J.; Veeck, L.; Rosenwaks, Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA 2001, 286, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Buyuk, E.; Veeck, L.; Zaninovic, N.; Xu, K.; Takeuchi, T.; Opsahl, M.; Rosenwaks, Z. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet 2004, 363, 837–840. [Google Scholar] [CrossRef]
- Oktay, K.; Turkcuoglu, I.; Rodriguez-Wallberg, K.A. Four spontaneous pregnancies and three live births following subcutaneous transplantation of frozen banked ovarian tissue: What is the explanation? Fertil. Steril. 2011, 95. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Cox, S.L.; Jenkin, G.; Findlay, J.; Trounson, A.; Shaw, J. Graft site and gonadotrophin stimulation influences the number and quality of oocytes from murine ovarian tissue grafts. Reproduction 2006, 131, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tao, Y.; Zhang, Y.; Li, Y.; Fang, F.; Liu, Y.; Cao, H.; Zhang, X.; Zhou, S. Follicle growth and oocyte development after ovary transplantation into back muscle of immune-intact adult castrated male mice. Reproduction 2010, 140, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Barboni, B.; Turriani, M.; Galeati, G.; Spinaci, M.; Bacci, M.L.; Forni, M.; Mattioli, M. Vascular endothelial growth factor production in growing pig antral follicles. Biol. Reprod. 2000, 63, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Jakeman, L.B.; Winer, J.; Bennett, G.L.; Altar, C.A.; Ferrara, N. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J. Clin. Investig. 1992, 89, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Koos, R.D. Potential relevance of angiogenic factors to ovarian physiology. Semin. Reprod. Endocrinol. 1989, 7, 29–40. [Google Scholar] [CrossRef]
- Neufeld, G.; Ferrara, N.; Schweigerer, L.; Mitchell, R.; Gospodarowicz, D. Bovine granulosa cells produce basic fibroblast growth factor. Endocrinology 1987, 121, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Wolner-Hanssen, P.; Hagglund, L.; Ploman, F.; Ramirez, A.; Manthorpe, R.; Thuring, A. Autotransplantation of cryopreserved ovarian tissue to the right forearm 4(1/2) years after autologous stem cell transplantation. Acta Obstet. Gynecol. Scand. 2005, 84, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Newton, H.; Gosden, R.G. Transplantation of cryopreserved human ovarian tissue results in follicle growth initiation in SCID mice. Fertil. Steril. 2000, 73, 599–603. [Google Scholar] [CrossRef]
- Oktay, K.; Newton, H.; Mullan, J.; Gosden, R.G. Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum. Reprod. 1998, 13, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- De Laporte, L.; des Rieux, A.; Tuinstra, H.M.; Zelivyanskaya, M.L.; de Clerck, N.M.; Postnov, A.A.; Preat, V.; Shea, L.D. Vascular endothelial growth factor and fibroblast growth factor 2 delivery from spinal cord bridges to enhance angiogenesis following injury. J. Biomed. Mater. Res. Part A 2011, 98, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.T.; Ding, Y.T.; Yan, X.G.; Wu, L.Y.; Li, Q.; Cheng, N.; Qiu, Y.D.; Zhang, M.Y. Angiogenic synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in an in vitro quantitative microcarrier-based three-dimensional fibrin angiogenesis system. World J. Gastroenterol. 2004, 10, 2524–2528. [Google Scholar] [CrossRef] [PubMed]
- Yoshiji, H.; Kuriyama, S.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Hicklin, D.J.; Huber, J.; Nakatani, T.; Tsujinoue, H.; Yanase, K.; et al. Synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in murine hepatocellular carcinoma. Hepatology 2002, 35, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Aydin, B.A.; Karlikaya, G. A technique for laparoscopic transplantation of frozen-banked ovarian tissue. Fertil. Steril. 2001, 75, 1212–1216. [Google Scholar] [CrossRef]
- Lee, R.K.; Ho, H.Y.; Yu, S.L.; Lu, C.H. Blastocyst development after cryopreservation and subcutaneous transplantation of mouse ovarian tissue. J. Assist. Reprod. Genet. 2005, 22, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Devine, K.; Connell, M.T.; Richter, K.S.; Ramirez, C.I.; Levens, E.D.; DeCherney, A.H.; Stillman, R.J.; Widra, E.A. Single vitrified blastocyst transfer maximizes liveborn children per embryo while minimizing preterm birth. Fertil. Steril. 2015, 103, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Frattarelli, J.L.; Leondires, M.P.; McKeeby, J.L.; Miller, B.T.; Segars, J.H. Blastocyst transfer decreases multiple pregnancy rates in in vitro fertilization cycles: A randomized controlled trial. Fertil. Steril. 2003, 79, 228–230. [Google Scholar] [CrossRef]
- Schnorr, J.; Oehninger, S.; Toner, J.; Hsiu, J.; Lanzendorf, S.; Williams, R.; Hodgen, G. Functional studies of subcutaneous ovarian transplants in non-human primates: Steroidogenesis, endometrial development, ovulation, menstrual patterns and gamete morphology. Hum. Reprod. 2002, 17, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.M.; Yan, J.; Li, R.; Li, M.; Yan, L.Y.; Wang, T.R.; Zhao, H.C.; Zhao, Y.; Yu, Y.; Qiao, J. Improvement in the quality of heterotopic allotransplanted mouse ovarian tissues with basic fibroblast growth factor and fibrin hydrogel. Hum. Reprod. 2013, 28, 2784–2793. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huang, Y.; Li, M.; Zhao, H.; Zhao, Y.; Li, R.; Yan, J.; Yu, Y.; Qiao, J. Effect of local basic fibroblast growth factor and vascular endothelial growth factor on subcutaneously allotransplanted ovarian tissue in ovariectomized mice. PLoS ONE 2015, 10, e0134035. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.J.; Wang, Y.; Zhang, L.; Xiao, Z.; Li, S.W. bFGF and VEGF improve the quality of vitrified-thawed human ovarian tissues after xenotransplantation to SCID mice. J. Assist. Reprod. Genet. 2016, 33, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.K.; Shin, D.H.; Kim, B.Y.; Yoon, S.Y.; Yoon, T.K.; Lee, W.S.; Chung, H.M.; Lee, D.R. Effect of human endothelial progenitor cell (EPC)- or mouse vascular endothelial growth factor-derived vessel formation on the survival of vitrified/warmed mouse ovarian grafts. Reprod. Sci. 2014, 21, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.; Peters, H. Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 1968, 17, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Summers, M.C.; McGinnis, L.K.; Lawitts, J.A.; Raffin, M.; Biggers, J.D. IVF of mouse ova in a simplex optimized medium supplemented with amino acids. Hum. Reprod. 2000, 15, 1791–1801. [Google Scholar] [CrossRef] [PubMed]



| Gonadotropins | Untreated Control | VEGF/FGF2 Treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| IU | PMF | PF | PAF | AF | PMF | PF | PAF | AF |
| 0 | 22.50 ± 10.65 | 17.33 ± 6.12 | 12.00 ± 2.97 ** | 12.00 ± 4.69 | 21.17 ± 21.08 | 13.67 ± 11.06 | 6.33 ± 3.88 | 6.33 ± 6.71 |
| 50 | 22.14 ± 14.37 | 12.29 ± 10.72 | 5.86 ± 7.31 | 6.71 ± 5.71 | 38.43 ± 17.60 * | 27.71 ± 13.78 * | 8.14 ± 7.38 | 13.14 ± 10.56 |
| 150 | 29.36 ± 13.50 | 13.91 ± 10.89 | 8.64 ± 5.92 | 6.73 ± 6.07 | 36.64 ± 22.35 | 24.09 ± 13.74 * | 11.73 ± 6.48 | 17.91 ± 12.19 * |
| 250 | 15.75 ± 12.09 | 9.75 ± 8.66 | 4.25 ± 3.86 | 4.50 ± 5.26 | 52.75 ± 13.84 ** | 39.25 ± 15.11 * | 18.00 ± 11.80 | 18.00 ± 16.75 |
| Oocyte Status | No. Oocytes—Control | No. Oocytes—VEGF/FGF2 |
|---|---|---|
| GV | 111 (69%) | 98 (51%) |
| MI | 18 (11%) | 28 (14%) |
| MII | 33 (20%) | 65 (35%) |
| Total No. oocytes | 162 | 191 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-H.; Hwu, Y.-M.; Lu, C.-H.; Chang, H.-H.; Hsieh, C.-E.; Lee, R.K.-K. VEGF and FGF2 Improve Revascularization, Survival, and Oocyte Quality of Cryopreserved, Subcutaneously-Transplanted Mouse Ovarian Tissues. Int. J. Mol. Sci. 2016, 17, 1237. https://doi.org/10.3390/ijms17081237
Li S-H, Hwu Y-M, Lu C-H, Chang H-H, Hsieh C-E, Lee RK-K. VEGF and FGF2 Improve Revascularization, Survival, and Oocyte Quality of Cryopreserved, Subcutaneously-Transplanted Mouse Ovarian Tissues. International Journal of Molecular Sciences. 2016; 17(8):1237. https://doi.org/10.3390/ijms17081237
Chicago/Turabian StyleLi, Sheng-Hsiang, Yuh-Ming Hwu, Chung-Hao Lu, Hsiao-Ho Chang, Cheng-En Hsieh, and Robert Kuo-Kuang Lee. 2016. "VEGF and FGF2 Improve Revascularization, Survival, and Oocyte Quality of Cryopreserved, Subcutaneously-Transplanted Mouse Ovarian Tissues" International Journal of Molecular Sciences 17, no. 8: 1237. https://doi.org/10.3390/ijms17081237
APA StyleLi, S.-H., Hwu, Y.-M., Lu, C.-H., Chang, H.-H., Hsieh, C.-E., & Lee, R. K.-K. (2016). VEGF and FGF2 Improve Revascularization, Survival, and Oocyte Quality of Cryopreserved, Subcutaneously-Transplanted Mouse Ovarian Tissues. International Journal of Molecular Sciences, 17(8), 1237. https://doi.org/10.3390/ijms17081237