Electrospun Poly(l-lactide)/Poly(ethylene glycol) Scaffolds Seeded with Human Amniotic Mesenchymal Stem Cells for Urethral Epithelium Repair
Abstract
:1. Introduction
2. Results
2.1. Fabrication of Poly(l-lactide)/Poly(ethylene glycol) (PLLA/PEG) Scaffolds
2.2. Isolation and Characterizations of Human Amniotic Mesenchymal Cells (hAMSCs)
2.3. Biocompatibility of PLLA/PEG Scaffolds
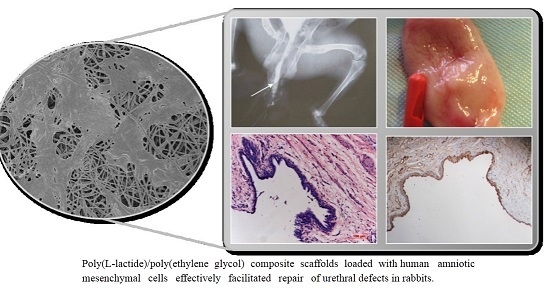
2.4. Urethrography Analysis and Morphological Observation
2.5. Histological Evaluation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of PLLA/PEG Scaffolds Using Electrospinning
4.3. Isolation of hAMSCs
4.4. Characterizations of hAMSCs
4.5. Culture of hAMSCs on PLLA/PEG Scaffolds
4.6. Cell Morphology
4.7. Preparation of hAMSC-PLLA/PEG Constructs for Implantation
4.8. Animal Surgeries
4.9. Postoperative Evaluations
4.10. H&E Staining
4.11. Immunohistochemistry
4.12. Statistics Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADMSCs | adipose-derived mesenchymal stem cells |
| α-MEM | α-Mininum essential medium |
| ANOVA | analysis of variance |
| BMSCs | bone marrow mesenchymal stem cells |
| CCK-8 | Cell Counting Kit-8 |
| DMEM | Dulbecco’s Modified Eagle’s medium |
| ECM | extracellular matrix |
| EDTA | ethylenediaminetetraacetic acid |
| FBS | fetal bovine serum |
| IACUC | Institutional Animal Care and Use Committee |
| IBMX | isobutylmethylxanthine |
| ITS | insulin-transferrin-selenious acid mix |
| hAMSCs | human amniotic mesenchymal cells |
| H&E | hematoxylin-eosin |
| HRP | horseradish peroxidase |
| MSCs | mesenchymal stem cells |
| PCL | polycaprolactone |
| PEG | poly(ethylene glycol) |
| PGA | polyglycolic acid |
| PLA | polylactide |
| PLGA | poly(l-lactide-co-glycolide) |
| PLLA | poly(l-lactide) |
| RUG | retrograde urethrogram |
| SEM | scanning electron microscopy |
References
- Marrocco, G.; Vallasciani, S.; Fiocca, G.; Calisti, A. Hypospadias surgery: A 10-year review. Pediatr. Surg. Int. 2004, 20, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.; Osman, N.; MacNeil, S. Developing tissue-engineered solutions for the treatment of extensive urethral strictures. Eur. Urol. 2013, 63, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl. Immunol. 2004, 12, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011, 32, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- El-Kassaby, A.; AbouShwareb, T.; Atala, A. Randomized comparative study between buccal mucosal and acellular bladder matrix grafts in complex anterior urethral strictures. J. Urol. 2008, 179, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Patterson, J.M.; Inman, R.D.; MacNeil, S.; Chapple, C.R. Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur. Urol. 2008, 53, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Raya-Rivera, A.; Esquiliano, D.R.; Yoo, J.J.; Lopez-Bayghen, E.; Soker, S.; Atala, A. Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet 2011, 377, 1175–1182. [Google Scholar] [CrossRef]
- Osman, N.I.; Patterson, J.M.; MacNeil, S.; Chapple, C.R. Long-term follow-up after tissue-engineered buccal mucosa urethroplasty. Eur. Urol. 2014, 66, 790–791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Guo, X.; Zhao, W.; Niu, G.; Mo, X.; Fu, Q. Application of wnt pathway inhibitor delivering scaffold for inhibiting fibrosis in urethra strictures: In vitro and in vivo study. Int. J. Mol. Sci. 2015, 16, 27659–27676. [Google Scholar] [CrossRef] [PubMed]
- De Kemp, V.; de Graaf, P.; Fledderus, J.O.; Ruud Bosch, J.L.; de Kort, L.M. Tissue engineering for human urethral reconstruction: Systematic review of recent literature. PLoS ONE 2015, 10, e0118653. [Google Scholar] [CrossRef] [PubMed]
- Orabi, H.; AbouShwareb, T.; Zhang, Y.; Yoo, J.J.; Atala, A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: A preclinical study. Eur. Urol. 2013, 63, 531–538. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, R.E.; Yoo, J.J.; Atala, A. Urethral replacement using cell seeded tubularized collagen matrices. J. Urol. 2002, 168, 1789–1793. [Google Scholar] [CrossRef]
- Yamzon, J.L.; Kokorowski, P.; Koh, C.J. Stem cells and tissue engineering applications of the genitourinary tract. Pediatr. Res. 2008, 63, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Jiang, F.; Zhao, L.; You, J.; Cao, X.; Li, Y. PLLA microalloys versus PLLA nanoalloys: Preparation, morphologies, and properties. ACS Appl. Mater. Interface 2012, 4, 3667–3675. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, J.; Jordan, R.; Luxenhofer, R. On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s. Biomaterials 2014, 35, 4848–4861. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, L.; Zhang, P.; Wang, X.; Chen, X.; Jing, X.; Su, Z. Surface modification of poly(l-lactic acid) to improve its cytocompatibility via assembly of polyelectrolytes and gelatin. Acta Biomater. 2006, 2, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ma, Y.; Wang, S.; Moran, P.M. A technique for preparing protein gradients on polymeric surfaces: Effects on PC12 pheochromocytoma cells. Biomaterials 2005, 26, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Y.; Fu, S.Z.; Ni, P.Y.; Peng, J.R.; Zheng, L.; Luo, F.; Liu, H.; Qian, Z.Y. Electrospun polylactide/poly(ethylene glycol) hybrid fibrous scaffolds for tissue engineering. J. Biomed. Mater. Res. A 2012, 100, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Cao, Y.L. Tissue engineering and stem cell application of urethroplasty: From bench to bedside. Urology 2012, 79, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guan, Y.; Liu, Y.; Zhu, M.; Li, T.; An, D.; Ou, L.; Che, Y.; Zhang, G.; Zhang, J.; et al. Fibrin glue with autogenic bone marrow mesenchymal stem cells for urethral injury repair in rabbit model. Tissue Eng. A 2012, 18, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.J.; Li, M.Y.; Huang, W.T.; Lu, M.H.; Hu, C.; Li, K.; Qiu, J.G.; Gao, X. Repair of urethral defects with polylactid acid fibrous membrane seeded with adipose-derived stem cells in a rabbit model. Connect. Tissue Res. 2015, 56, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhuang, Y.; Xiong, J.; Zhi, W.; Liu, L.; Wei, Q.; Han, P. Human umbilical mesenchymal stem cells-seeded bladder acellular matrix grafts for reconstruction of bladder defects in a canine model. PLoS ONE 2013, 8, e80959. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Prado, S.; Muinos-Lopez, E.; Hermida-Gomez, T.; Cicione, C.; Rendal-Vazquez, M.E.; Fuentes-Boquete, I.; de Toro, F.J.; Blanco, F.J. Human amniotic membrane as an alternative source of stem cells for regenerative medicine. Differentiation 2011, 81, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-S.; Park, S.-B.; Kim, H.-S.; Kang, J.-G.; Chae, J.-S.; Kang, K.-S. Isolation and characterization of equine amniotic membrane-derived mesenchymal stem cells. J. Vet. Sci. 2013, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Alviano, F.; Fossati, V.; Marchionni, C.; Arpinati, M.; Bonsi, L.; Franchina, M.; Lanzoni, G.; Cantoni, S.; Cavallini, C.; Bianchi, F.; et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev. Biol. 2007, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Miyoshi, S.; Ikegami, Y.; Hida, N.; Asada, H.; Togashi, I.; Suzuki, J.; Satake, M.; Nakamizo, H.; Tanaka, M.; et al. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ. Res. 2010, 106, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dehbari, N.; Han, W.; Huang, L.; Tang, Y. Electrospun multi-scale hybrid nanofiber/net with enhanced water swelling ability in rubber composites. Mater. Des. 2015, 86, 14–21. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, C.; Edmonds, L.; Yang, H.; Li, B. Microsol-electrospinning for controlled loading and release of water-soluble drugs in nanofibrous membranes. RSC Adv. 2014, 4, 43220–43226. [Google Scholar] [CrossRef]
- Xie, M.; Song, L.; Wang, J.; Fan, S.; Zhang, Y.; Xu, Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J. Surg. Res. 2013, 184, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.H.; Kim, H.K.; Akaike, T.; Kim, S.C. Adhesion and growth of endothelial cell on amphiphilic PU/PS IPN surface: Effect of amphiphilic balance and immobilized collagen. J. Biomed. Mater. Res. A 2002, 62, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Han, D.K.; Park, K.D.; Hubbell, J.A.; Kim, Y.H. Surface characteristics and biocompatibility of lactide-based poly(ethylene glycol) scaffolds for tissue engineering. J. Biomater. Sci. Polym. Ed. 1998, 9, 667–680. [Google Scholar] [PubMed]
- Li, B.; Ma, Y.; Wang, S.; Moran, P.M. Influence of carboxyl group density on neuron cell attachment and differentiation behavior: Gradient-guided neurite outgrowth. Biomaterials 2005, 26, 4956–4963. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Xu, Y.M.; Fu, Q.; Zhu, W.D.; Cui, L.; Chen, J. Evaluation of the biocompatibility and mechanical properties of naturally derived and synthetic scaffolds for urethral reconstruction. J. Biomed. Mater. Res. A 2010, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Atala, A. Urothelial cell culture: Stratified urothelial sheet and three-dimensional growth of urothelial structure. Methods Mol. Biol. 2013, 945, 383–399. [Google Scholar] [PubMed]







| Group | Number of Urethral Stricture and Urinary Fistula | Total Number | Incidence (%) | |
|---|---|---|---|---|
| + | − | |||
| A | 0 | 18 | 18 | 0.0 |
| B | 1 | 17 | 18 | 5.6 |
| C | 13 | 5 | 18 | 72.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Guo, Q.; Han, F.; Chen, C.; Ling, C.; Chen, W.; Li, B. Electrospun Poly(l-lactide)/Poly(ethylene glycol) Scaffolds Seeded with Human Amniotic Mesenchymal Stem Cells for Urethral Epithelium Repair. Int. J. Mol. Sci. 2016, 17, 1262. https://doi.org/10.3390/ijms17081262
Lv X, Guo Q, Han F, Chen C, Ling C, Chen W, Li B. Electrospun Poly(l-lactide)/Poly(ethylene glycol) Scaffolds Seeded with Human Amniotic Mesenchymal Stem Cells for Urethral Epithelium Repair. International Journal of Molecular Sciences. 2016; 17(8):1262. https://doi.org/10.3390/ijms17081262
Chicago/Turabian StyleLv, Xiaokui, Qianping Guo, Fengxuan Han, Chunyang Chen, Christopher Ling, Weiguo Chen, and Bin Li. 2016. "Electrospun Poly(l-lactide)/Poly(ethylene glycol) Scaffolds Seeded with Human Amniotic Mesenchymal Stem Cells for Urethral Epithelium Repair" International Journal of Molecular Sciences 17, no. 8: 1262. https://doi.org/10.3390/ijms17081262
APA StyleLv, X., Guo, Q., Han, F., Chen, C., Ling, C., Chen, W., & Li, B. (2016). Electrospun Poly(l-lactide)/Poly(ethylene glycol) Scaffolds Seeded with Human Amniotic Mesenchymal Stem Cells for Urethral Epithelium Repair. International Journal of Molecular Sciences, 17(8), 1262. https://doi.org/10.3390/ijms17081262