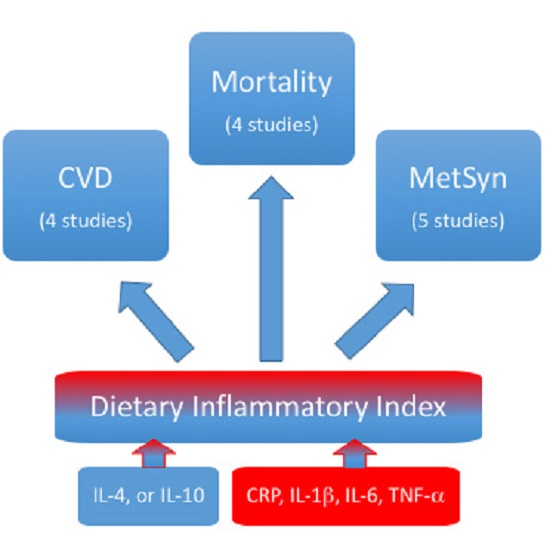
The Role of Dietary Inflammatory Index in Cardiovascular Disease, Metabolic Syndrome and Mortality
Abstract
:1. Introduction
2. Methods
3. The Dietary Inflammatory Index (DII) Score
4. Cardiovascular Disease and DII Score
5. Metabolic Syndrome and the DII Score
6. Mortality and the DII Score
7. The Relevance of the DII Score in the Association between Inflammation and Cardio-Metabolic Diseases
8. The DII Score in the Context of Healthy Dietary Patterns
9. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| CRP | C-Reactive Protein |
| DII | Dietary Inflammatory Index |
| FFQ | Food Frequency Questionnaire |
| HR | Hazard Ratio |
| IL | Interleukin |
| MedDiet | Mediterranean Diet |
| MetSyn | Metabolic Syndrome |
| OR | Odds Ratio |
| TNF-α | Tumor Necrosis Factor-α |
References
- Ross, R. Atherosclerosis–An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.D.; Lampe, J.W.; Kratz, M.; White, E. Lifestyle factors and inflammation: Associations by body mass index. PLoS ONE 2013, 8, e67833. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Abhari, S.; Luben, R.N.; Wareham, N.J.; Khaw, K.-T. Distribution and determinants of C-reactive protein in the older adult population: European Prospective Investigation into Cancer-Norfolk study. Eur. J. Clin. Investig. 2013, 43, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; David, H. (Eds.) Immunonutrition: Interactions of Diet, Genetics and Inflammation; CRC Press: Boca Raton, FL, USA, 2014.
- Calder, P.C.; Ahluwalia, N.; Albers, R.; Bosco, N.; Bourdet-Sicard, R.; Haller, D.; Holgate, S.T.; Jönsson, L.S.; Latulippe, M.E.; Marcos, A.; et al. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br. J. Nutr. 2013, 109, S1–S34. [Google Scholar] [CrossRef] [PubMed]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns and markers of systemic inflammation among Iranian women. J. Nutr. 2007, 137, 992–998. [Google Scholar] [PubMed]
- Casas, R.; Sacanella, E.; Urpi-Sarda, M.; Chiva-Blanch, G.; Ros, E.; Martínez-González, M.; Covas, M.-I.I.; Lamuela-Raventos, R.M.; Salas-Salvadó, J.; Fiol, M.; et al. The effects of the Mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Neale, E.P.; Batterham, M.J.; Tapsell, L.C. Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: A meta-analysis. Nutr. Res. 2016, 36, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Garcia-Arellano, A.; Estruch, R.; Marquez-Sandoval, F.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Viñoles, E.; Arós, F.; Herrera, C.; et al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur. J. Clin. Nutr. 2008, 62, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Salas-Salvadó, J.; Covas, M.I.; Toledo, E.; Andres-Lacueva, C.; Llorach, R.; et al. The Mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J. Nutr. 2012, 142, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R. Polyphenol intake from a mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A sub-study of The PREDIMED trial. Br. J. Clin. Pharmacol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Sun, Q.; Willett, W.C.; Eliassen, A.H.; Wu, K.; Pan, A.; Grodstein, F.; Hu, F.B. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am. J. Clin. Nutr. 2014, 99, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Herieka, M.; Faraj, T.A.; Erridge, C. Reduced dietary intake of pro-inflammatory Toll-like receptor stimulants favourably modifies markers of cardiometabolic risk in healthy men. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Marcason, W. What is the anti-inflammatory diet? J. Am. Diet. Assoc. 2010, 110, 1780. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Tabung, F.; Hébert, J.R. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014, 17, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hébert, J.R.; Rietzschel, E.R.; De Buyzere, M.L.; Langlois, M.; Debruyne, E.; Marcos, A.; Huybrechts, I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agalliu, I.; Hingle, M.; Hou, L.; Hurley, T.G.; Jiao, L.; et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Calzon, S.; Zalba, G.; Ruiz-Canela, M.; Shivappa, N.; Hébert, J.R.; Martínez, J.A.; Fito, M.; Gómez-Gracia, E.; Martínez-González, M.; Marti, A. Dietary inflammatory index and telomere length in subjects with a high cardiovascular disease risk from the PREDIMED-NAVARRA study: Cross-sectional and longitudinal analyses over 5 y. Am. J. Clin. Nutr. 2015, 102, 897–904. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, A.; Shivappa, N.; Jacka, F.N.; Kotowicz, M.A.; Kibbey, K.; Hébert, J.R.; Pasco, J.A. Pro-inflammatory dietary intake as a risk factor for CVD in men: A 5-year longitudinal study. Br. J. Nutr. 2015, 114, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arellano, A.; Ramallal, R.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Shivappa, N.; Schröder, H.; Hébert, J.R.; Ros, E.; Gómez-Gracia, E.; et al. Dietary Inflammatory Index and Incidence of Cardiovascular Disease in the PREDIMED Study. Nutrients 2015, 7, 4124–4138. [Google Scholar] [CrossRef] [PubMed]
- Ramallal, R.; Toledo, E.E.; Martínez-González, M.; Hernandez-Hernandez, A.; Garcia-Arellano, A.; Shivappa, N.; Hébert, J.R.; Ruiz-Canela, M.; Hernández-Hernández, A.; García-Arellano, A. Dietary inflammatory index and incidence of cardiovascular disease in the SUN cohort. PLoS ONE 2015, 10, e0135221. [Google Scholar] [CrossRef] [PubMed]
- Neufcourt, L.; Assmann, K.E.; Fezeu, L.K.; Touvier, M.; Graffouillère, L.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Hercberg, S.; Galan, P.; et al. Prospective association between the dietary inflammatory index and cardiovascular diseases in the Supplémentation en Vitamines et Minéraux Antioxydants (SU.VI.MAX) Cohort. J. Am. Heart Assoc. 2016, 5, e002735. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Shivappa, N.; Hurley, T.G.; Hébert, J.R. Association between previously diagnosed circulatory conditions and a dietary inflammatory index. Nutr. Res. 2016, 36, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sokol, A.; Wirth, M.D.; Manczuk, M.; Shivappa, N.; Zatonska, K.; Hurley, T.G.; Hébert, J.R. Association between the dietary inflammatory index, waist-to-hip ratio and metabolic syndrome. Nutr. Res. 2016. [Google Scholar] [CrossRef]
- Wirth, M.D.; Burch, J.; Shivappa, N.; Violanti, J.M.; Burchfiel, C.M.; Fekedulegn, D.; Andrew, M.E.; Hartley, T.A.; Miller, D.B.; Mnatsakanova, A.; et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J. Occup. Environ. Med. 2014, 56, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, A.M.; Toledo, E.; Rodriguez-Diez, M.C.; Gea, A.; Lopez-Iracheta, R.; Shivappa, N.; Hébert, J.R.; Martínez-González, M. Dietary indexes, food patterns and incidence of metabolic syndrome in a Mediterranean cohort: The SUN project. Clin. Nutr. 2015, 34, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Neufcourt, L.; Assmann, K.E.; Fezeu, L.K.; Touvier, M.; Graffouillère, L.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Hercberg, S.; Galan, P.; et al. Prospective association between the dietary inflammatory index and metabolic syndrome: Findings from the SU.VI.MAX study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Zazpe, I.; Shivappa, N.; Hébert, J.R.; Sánchez-Tainta, A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Lamuela-Raventós, R.M.; Rekondo, J.; et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvención con DIeta MEDiterránea) trial. Br. J. Nutr. 2015, 113, 984–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivappa, N.; Steck, S.E.; Hussey, J.R.; Ma, Y.; Hébert, J.R. Inflammatory potential of diet and all-cause, cardiovascular, and cancer mortality in National Health and Nutrition Examination Survey III Study. Eur. J. Nutr. 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.E.; Shivappa, N.; Tang, Y.; Mann, J.R.; Hébert, J.R. Association between diet-related inflammation, all-cause, all-cancer and cardiovascular disease mortality, with special focus on prediabetics: Findings from NHANES III. Eur. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Blair, C.K.; Prizment, A.E.; Jacobs, D.R.; Steck, S.E.; Hébert, J.R. Association between inflammatory potential of diet and mortality in the Iowa Women’s Health study. Eur. J. Nutr. 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Graffouillère, L.; Deschasaux, M.; Mariotti, F.; Neufcourt, L.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Latino-Martel, P.; Hercberg, S.; Galan, P.; et al. Prospective association between the Dietary Inflammatory Index and mortality: Modulation by antioxidant supplementation in the SU.VI.MAX randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Harris, H.; Wolk, A.; Hébert, J.R. Association between inflammatory potential of diet and mortality among women in the Swedish Mammography Cohort. Eur. J. Nutr. 2015, 55, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.; Panico, S.; Rubba, F.; Mattiello, A.; Chiodini, P.; Jossa, F.; Marotta, G.; Pauciullo, P.; Rubba, P. Obesity, overweight, and weight gain over adult life are main determinants of elevated hs-CRP in a cohort of Mediterranean women. Eur. J. Clin. Nutr. 2010, 64, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Polisecki, E.; Robertson, M.; Jahn, S.; Buckley, B.M.; de Craen, A.J.M.; Ford, I.; Jukema, J.W.; Macfarlane, P.W.; Packard, C.J.; et al. Unraveling the directional link between adiposity and inflammation: a bidirectional Mendelian randomization approach. J. Clin. Endocrinol. Metab. 2010, 95, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Timpson, N.J.; Nordestgaard, B.G.; Harbord, R.M.; Zacho, J.; Frayling, T.M.; Tybjærg-Hansen, A.; Smith, G.D. C-reactive protein levels and body mass index: Elucidating direction of causation through reciprocal Mendelian randomization. Int. J. Obes. 2011, 35, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Ramallal, R.; Toledo, E.; Martínez, J.A.; Shivappa, N.; Hebert, J.R.; Martínez-González, M.; Ruiz-Canela, M. Inflammatory potential of diet, weight gain and incidence of overweight/obesity: The SUN cohort. Clin. Nutr. 2016. under review. [Google Scholar]
- Moreno-Aliaga, M.; Campión, J.; Milagro, F.; Berjón, A.; Martínez, F. Adiposity and proinflammatory state: The chicken or the egg. Adipocytes 2005, 1, 1–16. [Google Scholar]
- Tang, Y.; Purkayastha, S.; Cai, D. Hypothalamic microinflammation: A common basis of metabolic syndrome and aging. Trend. Neurosci. 2015, 38, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Adler, A.I.; Sandhu, M.S.; Sharp, S.J.; Forouhi, N.G.; Erqou, S.; Luben, R.; Bingham, S.; Khaw, K.T.; Wareham, N.J. Association of C-reactive protein with type 2 diabetes: Prospective analysis and meta-analysis. Diabetologia 2009, 52, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Thompson, S.G.; Agewall, S.; Bergström, G.; Bickel, H.; Catapano, A.L.; Chien, K.-L.; de Groot, E.; Empana, J.-P.; Etgen, T.; et al. Inflammatory markers and extent and progression of early atherosclerosis: Meta-analysis of individual-participant-data from 20 prospective studies of the PROG-IMT collaboration. Eur. J. Prev. Cardiol. 2016, 23, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [PubMed]
- Wensley, F.; Gao, P.; Burgess, S.; Kaptoge, S.; di Angelantonio, E.; Shah, T.; Engert, J.C.; Clarke, R.; Davey-Smith, G.; Nordestgaard, B.G.; et al. Association between C reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ 2011, 342, d548. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Bias in causal estimates from Mendelian randomization studies with weak instruments. Stat. Med. 2011, 30, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving upstream to identify novel targets for atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification in the West Virginian Population. Int. J. Med. Sci. 2016, 13, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Carstensen, M.; Ouwens, D.M. Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Van Exel, E.; Gussekloo, J.; de Craen, A.J.M.; Frolich, M.; Bootsma-van der Wiel, A.; Westendorp, R.G.J. Low Production Capacity of Interleukin-10 Associates With the Metabolic Syndrome and Type 2 Diabetes : The Leiden 85-Plus Study. Diabetes 2002, 51, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; Seshasai, S.R.K.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.O.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef] [PubMed]
- I.-6R.M.R.A. (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: A mendelian randomisation analysis. Lancet 2012, 379, 1214–1224. [Google Scholar]
- Sarwar, N.; Butterworth, A.S.; Freitag, D.F.; Gregson, J.; Willeit, P.; Gorman, D.N.; Gao, P.; Saleheen, D.; Rendon, A.; Nelson, C.P.; et al. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet 2012, 379, 1205–1213. [Google Scholar] [PubMed]
- Mallat, Z.; Besnard, S.; Duriez, M.; Deleuze, V.; Emmanuel, F.; Bureau, M.F.; Soubrier, F.; Esposito, B.; Duez, H.; Fievet, C.; et al. Protective Role of Interleukin-10 in Atherosclerosis. Circ. Res. 1999, 85, e17–e24. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Murray, H.M.; Ford, I.; Trompet, S.; de Craen, A.J.M.; Jukema, J.W.; Stott, D.J.; McInnes, I.B.; Packard, C.J.; Westendorp, R.G.J.; et al. Circulating interleukin-10 and risk of cardiovascular events: A prospective study in the elderly at risk. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2338–2344. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Kim, P.H.; Lee, W.H.; Hirani, A.A. Interleukin-4, Oxidative Stress, Vascular Inflammation and Atherosclerosis. Biomol. Ther. 2010, 18, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Steffen, L.M. Nutrients, foods, and dietary patterns as exposures in research: A framework for food synergy. Am. J. Clin. Nutr. 2003, 78, 508S–513S. [Google Scholar] [PubMed]
- Guenther, P.M.; Reedy, J.; Krebs-Smith, S.M. Development of the Healthy Eating Index-2005. J. Am. Diet. Assoc. 2008, 108, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Guenther, P.M.; Casavale, K.O.; Reedy, J.; Kirkpatrick, S.I.; Hiza, H.A.B.; Kuczynski, K.J.; Kahle, L.L.; Krebs-Smith, S.M. Update of the Healthy Eating Index: HEI-2010. J. Acad. Nutr. Diet. 2013, 113, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Cross, A.J.; Subar, A.F.; Krebs-Smith, S.M.; Park, Y.; Powell-Wiley, T.; Hollenbeck, A.; Reedy, J. Comparison of 4 established DASH diet indexes: Examining associations of index scores and colorectal cancer. Am. J. Clin. Nutr. 2013, 98, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Diet Quality as Assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2015, 115, 780–800. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.; Salas-Salvadó, J.; Estruch, R.; Corella, D.D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights from the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ 2015, 5, e008222. [Google Scholar] [CrossRef] [PubMed]
- Mancini, J.G.; Filion, K.B.; Atallah, R.; Eisenberg, M.J. Systematic Review of the Mediterranean Diet for Long-Term Weight Loss. Am. J. Med. 2016, 129, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Shirani, F.; Chitsazi, M.J.; Salehi-Abargouei, A. The effect of dietary approaches to stop hypertension (DASH) diet on weight and body composition in adults: A systematic review and meta-analysis of randomized controlled clinical trials. Obes. Rev. 2016, 17, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martínez-González, M.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Chiva-Blanch, G.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; Lapetra, J.; et al. Effect of a high-fat mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2016. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committe. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. In Proceedings of the USDA and US Department of Health and Human Services; Dietary Guidelines Advisory Committe: Washington, DC, USA, 2015. [Google Scholar]
- Wirth, M.D.; Hébert, J.R.; Shivappa, N.; Hand, G.A.; Hurley, T.G.; Drenowatz, C.; McMahon, D.; Shook, R.P.; Blair, S.N. Anti-inflammatory Dietary Inflammatory Index scores are associated with healthier scores on other dietary indices. Nutr. Res. 2016, 36, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 84, 1489–1497. [Google Scholar] [PubMed]
- Hermsdorff, H.H.M.; Zulet, M.A.; Puchau, B.; Martínez, J.A. Fruit and vegetable consumption and proinflammatory gene expression from peripheral blood mononuclear cells in young adults: A translational study. Nutr. Metab. 2010, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.M.; Zulet, M.Á.; Abete, I.; Martínez, J.A. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur. J. Nutr. 2011, 50, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; López-Uriarte, P.; Bulló, M.; Ros, E.; Cabré-Vila, J.J.; Salas-Salvadó, J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Griffith, J.A.; Chasan-Taber, L.; Olendzki, B.C.; Jackson, E.; Stanek, E.J.; Li, W.; Pagoto, S.L.; Hafner, A.R.; Ockene, I.S. Association between dietary fiber and serum C-reactive protein. Am. J. Clin. Nutr. 2006, 83, 760–766. [Google Scholar] [PubMed]
- Nettleton, J.A.; Steffen, L.M.; Mayer-Davis, E.J.; Jenny, N.S.; Jiang, R.; Herrington, D.M.; Jacobs, D.R. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2006, 83, 1369–1379. [Google Scholar] [PubMed]
- Julia, C.; Meunier, N.; Touvier, M.; Ahluwalia, N.; Sapin, V.; Papet, I.; Cano, N.; Hercberg, S.; Galan, P.; Kesse-Guyot, E. Dietary patterns and risk of elevated C-reactive protein concentrations 12 years later. Br. J. Nutr. 2013, 110, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effe of Olive Oil on Markers of Inflammation and Endothelial Function—A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [PubMed]
- Van Woudenbergh, G.J.; Theofylaktopoulou, D.; Kuijsten, A.; Ferreira, I.; van Greevenbroek, M.M.; van der Kallen, C.J.; Schalkwijk, C.G.; Stehouwer, C.D.; Ocke, M.C.; Nijpels, G.; et al. Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: The Cohort study on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. Am. J. Clin. Nutr. 2013, 98, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Chan, A.T.; Willett, W.C.; Giovannucci, E.L. Development and validation of an empirical Dietary Inflammatory Index. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; Bassett, J.K.; Shivappa, N.; Hébert, J.R.; English, D.R.; Giles, G.G.; Severi, G. Dietary inflammatory index, Mediterranean diet score, and lung cancer: A prospective study. Cancer Causes Control 2016, 27, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hébert, J.R.; Rosato, V.; Rossi, M.; Montella, M.; Serraino, D.; la Vecchia, C. Dietary inflammatory index and ovarian cancer risk in a large Italian case-control study. Cancer Causes Control 2016, 27, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hébert, J.R.; Rosato, V.; Serraino, D.; la Vecchia, C. Inflammatory potential of diet and risk of laryngeal cancer in a case-control study from Italy. Cancer Causes Control 2016, 27, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Zucchetto, A.; Gini, A.; Shivappa, N.; Hébert, J.R.; Stocco, C.; dal Maso, L.; Birri, S.; Serraino, D.; Polesel, J. Dietary inflammatory index and prostate cancer survival. Int. J. Cancer 2016. [Google Scholar] [CrossRef] [PubMed]


| Study Name | Design | # Food Parameters | Follow-up 1 (Years) | N Total (CVD Cases) | Groups | Adjusted Relative Risk (95% CI) | Covariables |
|---|---|---|---|---|---|---|---|
| GOS 2 [27] | Cohort | 22 using FFQ | 5 | 1363 (76) | Negative DII (ref) vs. Positive DII | OR = 2.00 (1.01–3.96) | Family history of CVD, blood pressure, sedentary, diabetes, smoking, waist circumference, age, total energy intake |
| PREDIMED 3 [28] | Cohort | 32 using FFQ | 4.7 | 7216 (277) | Quartile 1 (ref) vs. Quartile 4 | HR = 1.73 (1.15–2.60) | Age, sex, overweight/obesity, waist-to-height ratio, total energy intake, smoking status, diabetes, hypertension, dyslipidemia, family history of premature cardiovascular disease, physical activity, educational level, intervention group, center |
| SUN 4 [29] | Cohort | 28 using FFQ | 8.9 | 18,794 (117) | Quartile 1 (ref) vs. Quartile 4 | HR = 2.03 (1.06–3.88) | Age, sex, hypertension, dyslipidaemia, diabetes, smoking status, family history of cardiovascular disease, total energy intake, physical activity, body mass index, educational level, other cardiovascular diseases, special diet at baseline, snacking, average time sitting, average time spent watching television |
| SU.VI.MAX 5 [30] | Cohort | 36 using 24-h dietary records | 11.4 | 7743 (292) | Quartile 1 (ref) vs. Quartile 4 | HR = 1.16 (0.79–1.69) | Sex, energy intake, supplementation group, number of 24-h records, education level, marital status, smoking status, physical activity, body mass index |
| 11.4 | 7602 (93) | Quartile 1 (ref) vs. Quartile 4 | HR = 2.26 * (1.08–4.71) | ||||
| NHANES 6 [31] | Cross-sectional | 27 using 24-h dietary records | NA | 15,693 (1734) | Quartile 1 (ref) vs. Quartile 4 | OR = 1.30 (1.06–1.58) | Family member smoking status, personal smoking status, age, body mass index |
| Study Name | Design | # Food Parameters | Follow-up (Years) | N Total (Cases) | Groups | Adjusted Relative Risk (95% CI) | Covariables |
|---|---|---|---|---|---|---|---|
| PONS 2 [32] | Cross-sectional | 22 using FFQ | NA | 3862 (1159) | Quartile 1 (ref) vs. Quartile 4 | OR = 0.96 (0.77–1.19) | Body mass index, age |
| BCOPS 3 [33] | Cross-sectional | Not reported | NA | 464 (125) | Quartile 1 (ref) vs. Quartile 4 | OR = 0.87 (0.46–1.63) | Age, sex |
| SUN 4 [34] | Cohort | 28 using FFQ | 8.3 | 6851 (346) | Quintile 1 (ref) vs. Quintile 5 | HR * = 0.86 (0.60–1.23) | Age, sex, smoking, alcohol consumption, snacking between main meals, use of special diets, television watching, physical activity, changes in weight over the last 5 years prior, body mass index |
| SU.VI.MAX 5 [35] | Cohort | 36 using 24-h dietary records | 12.4 | 3726 (524) | Quartile 1 (ref) vs. Quartile 4 | HR = 1.39 (1.01–1.92) | Age, sex, supplementation group, number of 24 h records, energy intake, education level, smoking status, physical activity, body mass index |
| Study Name | Design | # Food Parameters | Follow-up (Years) | N Total (Cases) | Groups | Adjusted Relative Risk (95% CI) | Covariables |
|---|---|---|---|---|---|---|---|
| NHANES 1 III [37] | Cohort | 27 using 24-h dietary records | 13.5 | 12,438 (2795) | Tertile 1 (ref) vs. Tertile 3 | HR = 1.34 (1.19, 1.51) | Age, sex, race, diabetes status, hypertension, physical activity, BMI, poverty index, smoking |
| NHANES 1 III [38] | Cohort | 27 using 24-h dietary records | NA | 2681 (896) | Tertile 1 (ref) vs. Tertile 3 | HR = 1.39 (1.13, 1.72) | Age, sex, race, HbA1C, current smoking, physical activity, body mass index, systolic blood pressure |
| Iowa Women’s Health study [39] | Cohort | 37 using FFQ | 20.7 | 37,525 (17,793) | Quartile 1 (ref) vs. Quartile 4 | HR = 1.08 (1.03–1.13) | Age, body mass index, smoking status, pack-years of Smoking, hormone replacement therapy use, education, diabetes, hypertension, heart disease, cancer, total energy intake |
| SU.VI.MAX 2 [40] | Cohort | 36 using 24-h dietary records | 1.24 | 8089 (207) | Tertile 1 (ref) vs. Tertile 3 | HR * = 2.10 (1.15–3.84) | Age, sex, intervention group, number of 24-hour dietary records, body mass index, physical activity, smoking status, educational level, family history of cancer in first-degree relatives, family history of CVD in first-degree relatives, energy intake without alcohol, and alcohol intake |
| HR ** = 1.09 (0.67–1.77) | |||||||
| Swedish Mammography Cohort [41] | Cohort | 27 using FFQ | 15 | 33,747 (7095) | Quintile 1 (ref) vs. Quintile 5 | HR = 1.41 (1.21–1.64) | Age, energy intake, body mass index, education, smoking status, physical activity, alcohol intake |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Canela, M.; Bes-Rastrollo, M.; Martínez-González, M.A. The Role of Dietary Inflammatory Index in Cardiovascular Disease, Metabolic Syndrome and Mortality. Int. J. Mol. Sci. 2016, 17, 1265. https://doi.org/10.3390/ijms17081265
Ruiz-Canela M, Bes-Rastrollo M, Martínez-González MA. The Role of Dietary Inflammatory Index in Cardiovascular Disease, Metabolic Syndrome and Mortality. International Journal of Molecular Sciences. 2016; 17(8):1265. https://doi.org/10.3390/ijms17081265
Chicago/Turabian StyleRuiz-Canela, Miguel, Maira Bes-Rastrollo, and Miguel A. Martínez-González. 2016. "The Role of Dietary Inflammatory Index in Cardiovascular Disease, Metabolic Syndrome and Mortality" International Journal of Molecular Sciences 17, no. 8: 1265. https://doi.org/10.3390/ijms17081265
APA StyleRuiz-Canela, M., Bes-Rastrollo, M., & Martínez-González, M. A. (2016). The Role of Dietary Inflammatory Index in Cardiovascular Disease, Metabolic Syndrome and Mortality. International Journal of Molecular Sciences, 17(8), 1265. https://doi.org/10.3390/ijms17081265