Sanguinaria canadensis: Traditional Medicine, Phytochemical Composition, Biological Activities and Current Uses
Abstract
:1. Introduction
2. Botany of S. canadensis
3. Historical Uses of S. canadensis
3.1. Traditional Native American Uses of S. canadensis
3.2. Early Western Use of S. canadensis
4. Phytochemicals in S. canadensis
4.1. Identification of Alkaloids in S. canadensis
4.2. Alkaloid Distribution in Different Plant Tissues of S. canadensis
4.3. Rhizome Alkaloid Composition and Variation
4.4. Other Factors Affecting S. canadensis Rhizome Alkaloid Concentrations
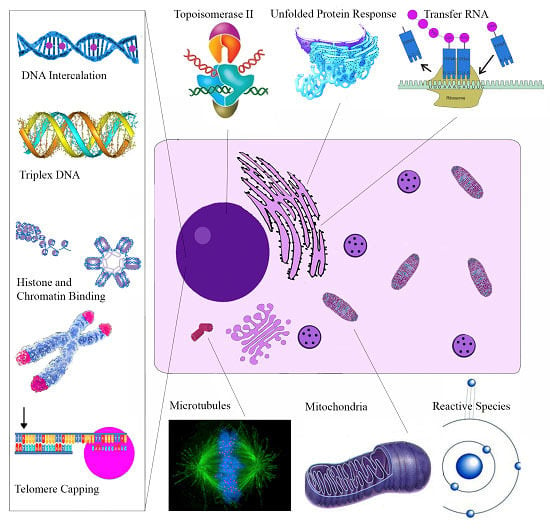
5. Biological Activities of S. canadensis Alkaloids
5.1. Anti-Cancer Effects of S. canadensis Alkaloids
5.1.1. Sanguinarine
5.1.2. Chelerythrine
5.1.3. Minor Quaternary Benzophenanthridine Alkaloids (QBAs)
5.1.4. Protopin Alkaloids
5.2. Cardiovascular Effects of S. canadensis Alkaloids
5.3. Anti-Inflammatory Actions of S. canadensis Alkaloids
5.4. Anti-Infectious Effects of S. canadensis Alkaloids
5.5. Other Biological Activities of S. canadensis Alkaloids
6. Current Uses of S. canadensis
6.1. Agriculture and Aquaculture Feed Supplement
6.2. Veterinary Uses
6.3. Dental Antibacterial Treatment
6.4. Homeopathic Treatment
6.5. Use in Cancer Therapy
6.5.1. Mohs Paste
6.5.2. Salve Chemosurgery
7. Future Application and Risks of Using S. canadensis
8. Conclusions
Conflicts of Interest
References
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.J. American Indian Medicine; University of Oklahoma Press: Norman, OK, USA, 2013. [Google Scholar]
- Mazzio, E.A.; Soliman, K.F. In vitro screening for the tumoricidal properties of international medicinal herbs. Phytother. Res. 2009, 23, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Slavik, J.; Slavikova, L. Alkaloide der mohngewächse (papaveraceae) XVII. Über neue alkaloide aus Sanguinaria canadensis L. Collect. Czechoslov. Chem. Commun. 1960, 25, 1667–1675. [Google Scholar] [CrossRef]
- Damm, D.D.; Fantasia, J.E. White patch of maxillary vestibule. Sanguinarine-associated leukoplakia. Gen. Dent. 2002, 50, 466. [Google Scholar] [PubMed]
- Lu, J.J.; Bao, J.L.; Chen, X.P.; Huang, M.; Wang, Y.T. Alkaloids isolated from natural herbs as the anticancer agents. Evid. Based Complement. Altern. Med. 2012, 2012, 485042. [Google Scholar] [CrossRef] [PubMed]
- Bambagiotti-Alberti, M.; Pinzauti, S.; Moneti, G.; Gratteri, P.; Coran, S.A.; Vincieri, F.F. Characterization of Sanguinaria canadensis L. Fluid extract by fab mass spectrometry. J. Pharm. Biomed. Anal. 1991, 9, 1083–1087. [Google Scholar] [CrossRef]
- Malikova, J.; Zdarilova, A.; Hlobilkova, A. Effects of sanguinarine and chelerythrine on the cell cycle and apoptosis. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2006, 150, 5–12. [Google Scholar] [CrossRef]
- Miao, F.; Yang, X.J.; Zhou, L.; Hu, H.J.; Zheng, F.; Ding, X.D.; Sun, D.M.; Zhou, C.D.; Sun, W. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Nat. Prod. Res. 2011, 25, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Mackraj, I.; Govender, T.; Gathiram, P. Sanguinarine. Cardiovasc. Ther. 2008, 26, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Schmeller, T.; Latz-Bruning, B. Modes of action of allelochemical alkaloids: Interaction with neuroreceptors, DNA, and other molecular targets. J. Chem. Ecol. 1998, 24, 1881–1937. [Google Scholar] [CrossRef]
- Saeed, S.; Gilani, A.; Majoo, R.; Shah, B. Anti-thrombotic and anti-inflammatory activities of protopine. Pharmacol. Res. 1997, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kosina, P.; Walterova, D.; Ulrichova, J.; Lichnovsky, V.; Stiborova, M.; Rydlova, H.; Vicar, J.; Krecman, V.; Brabec, M.J.; Simanek, V. Sanguinarine and chelerythrine: Assessment of safety on pigs in ninety days feeding experiment. Food Chem. Toxicol. 2004, 42, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hannah, J.J.; Johnson, J.D.; Kuftinec, M.M. Long-term clinical evaluation of toothpaste and oral rinse containing sanguinaria extract in controlling plaque, gingival inflammation, and sulcular bleeding during orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 1989, 96, 199–207. [Google Scholar] [CrossRef]
- Jäggi, R.; Würgler, U.; Grandjean, F.; Weiser, M. Dual inhibition of 5-lipoxygenase/cyclooxygenase by a reconstituted homeopathic remedy; possible explanation for clinical efficacy and favourable gastrointestinal tolerability. Inflamm. Res. 2004, 53, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.L.; Brewer, J.D. Black salve and bloodroot extract in dermatologic conditions. Cutis 2015, 95, 309–311. [Google Scholar] [PubMed]
- Vlachojannis, C.; Magora, F.; Chrubasik, S. Rise and fall of oral health products with canadian bloodroot extract. Phytother. Res. 2012, 26, 1423–1426. [Google Scholar] [CrossRef] [PubMed]
- Eastman, K.L.; McFarland, L.V.; Raugi, G.J. A review of topical corrosive black salve. J. Altern. Complement. Med. 2014, 20, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Laub, D.R., Jr. Death from metastatic basal cell carcinoma: Herbal remedy or just unlucky? J. Plast. Reconstr. Aesth. Surg. 2008, 61, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Salmore, A.K.; Hunter, M.D. Environmental and genotypic influences on isoquinoline alkaloid content in Sanguinaria canadensis. J. Chem. Ecol. 2001, 27, 1729–1747. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.C.; Bell, C.R.; Boulware, R.T. Geographic variation in alkaloid content of Sanguinaria canadensis (Papaveraceae). Rhodora 1990, 92, 57–69. [Google Scholar]
- Gaziano, R.; Moroni, G.; Buè, C.; Miele, M.T.; Sinibaldi-Vallebona, P.; Pica, F. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: Evidence and perspectives. World J. Gastrointest. Oncol. 2016, 8, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Senchina, D.S.; Flinn, G.N.; McCann, D.A.; Kohut, M.L.; Shearn, C.T. Bloodroot (Sanguinaria canadensis L., papaveraceae) enhances proliferation and cytokine production by human peripheral blood mononuclear cells in an in vitro model. J. Herbs Spices Med. Plants 2009, 15, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Debiton, E.; Madelmont, J.C.; Legault, J.; Barthomeuf, C. Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother. Pharmacol. 2003, 51, 474–482. [Google Scholar] [PubMed]
- Karp, J.M.; Rodrigo, K.A.; Pei, P.; Pavlick, M.D.; Andersen, J.D.; McTigue, D.J.; Fields, H.W.; Mallery, S.R. Sanguinarine activates polycyclic aromatic hydrocarbon associated metabolic pathways in human oral keratinocytes and tissues. Toxicol. Lett. 2005, 158, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Keith, C.T.; Borisy, A.A.; Stockwell, B.R. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 2005, 4, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ernst, W.R. The genera of papaveraceae and fumariaceae in the Southeastern United States. J. Arnold Arbor. 1962, 43, 315–343. [Google Scholar]
- Nieuwland, J.A. Notes on the seedlings of bloodroot. Am. Midl. Nat. 1910, 1, 199–203. [Google Scholar] [CrossRef]
- Parkinson, J. Theatrum Botanicum: The Theater of Plants, or, an Herball of a Large Extent; Tho. Cotes: London, UK, 1640; pp. 324–334. [Google Scholar]
- Plukenet, L. Almagestum Botanicum; Sumptibus Auctoris: London, UK, 1696; pp. 279–280. [Google Scholar]
- Felter, H.W.; Lloyd, J.U. Sanguinaria (u.S.P.)–Sanguinaria. In King’s American Dispensatory; Ohio Valley Co.: Cincinnatti, OH, USA, 1898; pp. 1708–1714. [Google Scholar]
- Dillen, J.J. Hortus Elthamensis seu Plantarum Rariorum quas in Horto suo Elthami in Cantio, Coluit... Jacobus Sherard,... Delineationes et Descriptiones... Auctore Johanne Jacobo Dillenio md; Sumptibus Auctoris: London, UK, 1732; pp. 334–336. [Google Scholar]
- Linnaeus, C. Species Plantarum; Laurentii Salvii: Stockholm, Sweden, 1753; p. 505. [Google Scholar]
- Rafinesque, C.S. Manual of the Medical Botany of the United States of North America; Atkinson & Alexander: Philadelphia, PA, USA, 1830; Volume 2, pp. 78–81. [Google Scholar]
- Lyon, D.L. Bee pollination of facultatively xenogamous Sanguinaria canadensis L. Bull. Torrey Bot. Club 1992, 119, 368–375. [Google Scholar] [CrossRef]
- Beattie, A.J.; Culver, D.C. The guild of myrmecochores in the herbaceous Flora of West Virginia forests. Ecology 1981, 62, 107–115. [Google Scholar] [CrossRef]
- Pudlo, R.J.; Beattie, A.J.; Culver, D.C. Population consequences of changes in an ant-seed mutualism in Sanguinaria canadensis. Oecologia 1980, 46, 32–37. [Google Scholar] [CrossRef]
- Gomez, C.; Espadaler, X. Myrmecochorous dispersal distances: A world survey. J. Biogeogr. 1998, 25, 573–580. [Google Scholar] [CrossRef]
- Lobstein, M.B.; Rockwood, L.L. Influence of elaiosome removal on germination in five ant-dispersed plant species. Va. J. Sci. 1993, 44, 59–72. [Google Scholar]
- Porter, S.D.; Savignano, D.A. Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology 1990, 71, 2095–2106. [Google Scholar] [CrossRef]
- Zettler, J.A.; Spira, T.P.; Allen, C.R. Ant–seed mutualisms: Can red imported fire ants sour the relationship? Biol. Conserv. 2001, 101, 249–253. [Google Scholar] [CrossRef]
- Heithaus, E.R. Seed predation by rodents on three ant-dispersed plants. Ecology 1981, 136–145. [Google Scholar] [CrossRef]
- Grieve, M.; Leyel, C.F. A Modern Herbal the Medicinal Culinary Cosmetic and Economic Properties Cultivation and Folk-Lore of Herbs, Grases Fungi, Shrubs & Trees with All Their Modern Uses; Harcourt, Brace & Co.: New York, NY, USA, 1931. [Google Scholar]
- Fern, K. Plants for a Future: The Species Database. Available online: http://www.ibiblio.org/pfaf/database/latinS.html (accessed on 8 May 2016).
- Marino, P.C.; Eisenberg, R.M.; Cornell, H.V. Influence of sunlight and soil nutrients on clonal growth and sexual reproduction of the understory perennial herb Sanguinaria canadensis L. J. Torrey Bot. Soc. 1997, 219–227. [Google Scholar] [CrossRef]
- Boothroyd-Roberts, K.; Gagnon, D.; Truax, B. Hybrid poplar plantations are suitable habitat for reintroduced forest herbs with conservation status. SpringerPlus 2013, 2, 507. [Google Scholar] [CrossRef] [PubMed]
- Salmore, A.K.; Hunter, M.D. Elevational trends in defense chemistry, vegetation, and reproduction in Sanguinaria canadensis. J. Chem. Ecol. 2001, 27, 1713–1727. [Google Scholar] [CrossRef] [PubMed]
- Erichsen-Brown, C. Medicinal and other Uses of North American Plants: A Historical Survey with Special Reference to the Eastern Indian Tribes; Courier Corporation: North Chelmsford, MA, USA, 2013. [Google Scholar]
- Chamberlain, A.F. Algonkian words in american english: A study in the contact of the white man and the Indian. J. Am. Folk. 1902, 15, 240–267. [Google Scholar] [CrossRef]
- Strachey, W. The Historie of Travaile into Virginia Britannia; Hakluyt Society: London, UK, 1849; p. 64. [Google Scholar]
- Gilmore, M.R. Uses of Plants by the Indians of the Missouri River Region; University of Nebraska Press: Lincoln, NE, USA, 1991; pp. 44–45. [Google Scholar]
- Herrick, J.W.; Snow, D.R. Iroquois Medical Botany; Syracuse University Press: Syracuse, NY, USA, 1995; pp. 127–128. [Google Scholar]
- Smith, H.H. Ethnobotany of the Ojibwe Indians; Order of the Board of Trustees: Milwaukee, WI, USA, 1932; pp. 377–378. [Google Scholar]
- Smith, H.H. Ethnobotany of the Forest Potawatomi Indians; AMS Press Inc.: New York, NY, USA, 1933; pp. 175–326. [Google Scholar]
- Tantaquidgeon, G. A Study of Delaware Indian Medicine Practice and Folk Beliefs; Commonwealth of Pennsylvania, Departmet of Public Instruction, Pennsylvania Historical Commission: Harrisburg, PA, USA, 1942; p. 32. [Google Scholar]
- Speck, F.G. Medicine practices of the Northeastern Algonquians; International Congress of Americanists: Washington, DC, USA, 1917; p. 318. [Google Scholar]
- Densmore, F. Uses of plants by the Chippewa Indians. Bur. Am. Ethnol. Bull. 1928, 44, 275–397. [Google Scholar]
- Mechling, W.H. The malecite Indians, with notes on the Micmacs (concluded). Anthropologica 1959, 8, 161–274. [Google Scholar] [CrossRef]
- Rousseau, J. Ethnobotanique abénaklse. Arch. Folk. 1947, 11, 145–182. [Google Scholar]
- Sollenberger, R.R. Rappahannock field notes. Am. Philos. Soc. 1940. [Google Scholar]
- Smith, H.H. Ethnobotany of the Meskwaki Indians; Public Museum of the City of Milwaukee: Milwaukee, WI, USA, 1928. [Google Scholar]
- Chandler, R.F. Vindication of maritime Indian herbal remedies. J. Ethnopharmacol. 1983, 9, 323–327. [Google Scholar] [CrossRef]
- Hunter, J.D. Manners and Customs of several Indian Tribes Located West of the Mississippi: Including Some Account of the Soil, Climate, and Vegetable Productions, and the Indian Materia Medica: To Which Is Prefixed the History of the Author’s Life during a Residence of Several Years among Them; Ross & Haines: Minneapolis, PA, USA, 1823; p. 384. [Google Scholar]
- Krochmal, A. Medicinal plants and Appalachia. Econ. Bot. 1968, 22, 332–337. [Google Scholar] [CrossRef]
- Downey, W. An Investigation of the Properties of the Sanguinaria canadensis; or Puccoon; Eaken & Mecum.: Philadelphia, PA, USA, 1803; pp. 23–25. [Google Scholar]
- Gibb, G.D. The Sanguinaria canadensis: Its natural history, properties, and medical uses. BMJ 1860, 4, 104–107. [Google Scholar] [CrossRef]
- Allen, J.A. Remarks on the treatment of tracheitis, or croup. Boston Med. Surg. J. 1845, 33, 389–392. [Google Scholar] [CrossRef]
- Eberle, J. A Treatise of the Materia Medica and Therapeutics; Grigg & Elliot: Philadelphia, PA, USA, 1834; Volume 2, pp. 73–76. [Google Scholar]
- Leonard, J. On the use of Sanguinaria. Boston Med. Surg. J. 1845, 32, 457–459. [Google Scholar] [CrossRef]
- Leopold, D. A history of rhinology in North America. Otolaryngol. Head Neck Surg. 1996, 115, 283–297. [Google Scholar] [CrossRef]
- Barton, W.P.C. Vegetable Materia Medica of the United States, or, Medical Botany: Containing a Botanical, General, and Medical History, of Medicinal Plants Indigenous to the United States: Illustrated by Colored Engravings, Made after Original Drawings from Nature, Done by the Author; M. Carey & Son: Philadelphia, PA, USA, 1817; pp. 30–42. [Google Scholar]
- Thatcher, J. The American New Dispensatory, 2nd ed.; Thomas B. Wait & Co. and C. Williams: Boston, MA, USA, 1813; pp. 201–204. [Google Scholar]
- Bartholow, R. A Practical Treatise on Materia Medica and Therapeutics; D. Appleton: New York, NY, USA, 1888; pp. 359–361. [Google Scholar]
- William, T. Sanguinarine and its salts: On the medicinal powers of sanguinarine and its salts. Boston Med. Surg. J. 1832, 6, 245–248. [Google Scholar] [CrossRef]
- Mackentosh, J.; Holderwell, S. Receipts for the Cure of Most Diseases to the Human Family; U.S. National Library of Medicine: New York, NY, USA, 1827; p. 9. [Google Scholar]
- Smith, D.B. On Sanguinaria canadensis. Boston Med. Surg. J. 1832, 5, 393–395. [Google Scholar] [CrossRef]
- Ellingwood, F.; Lloyd, J.U. American Materia Medica, Therapeutics and Pharmacognosy: Developing the Latest Acquired Knowledge of Drugs, and Especially of the Direct Action of Single Drugs upon Exact Conditions of Disease, With Especial Reference to the Therapeutics of the Plant Drugs of the Americas; Ellingwoods’ Therapeutist: Evanston, IL, USA, 1915; pp. 386–387. [Google Scholar]
- Stillé, A. Therapeutics and Materia Medica; Blanchard and Lea: Philadelphia, PA, USA, 1874; Volume 2, pp. 454–457. [Google Scholar]
- Cook, W. The Physiomedical Dispensatory; Wm. H. Cook: Cincinnati, OH, USA, 1869; pp. 466–467. [Google Scholar]
- Beach, W. The American Practice of Medicine; Betts & Anstice: New York, NY, USA, 1833; Volume 2, pp. 259–261. [Google Scholar]
- Fell, J.W. A Treatise on Cancer, and Its Treatment; John Churchill: London, UK, 1857; pp. 59–63. [Google Scholar]
- Shaw, A.D.M.C.; Moore, C.H.; Henry, M. Editorial. West. Lancet Mon. J. Pract. Med. Surg. 1857, 18, 541–542. [Google Scholar]
- Hooper, S.N.; Chandler, R.F. Herbal remedies of the maritime Indians: Phytosterols and triterpenes of 67 plants. J. Ethnopharmacol. 1984, 10, 181–194. [Google Scholar] [CrossRef]
- Dana, J.F. An account of some experiments on the root of the Sanguinaria canadensis. N. Y. Med. Phys. J. 1827, 6, 218–222. [Google Scholar] [CrossRef]
- Probst, Q.M. Beschreibung und Dartsellungsweise einiger bei der Analyse de Chelidonium majus neu aufgefundenen Stoffe. Justus Liebigs Ann. Chem. 1839, 29, 113. [Google Scholar]
- Konig, G. Über Papaveraceen-Alkaloïde. Arch. Pharm. 1893, 231, 177. [Google Scholar]
- Gadamer, J.; Stichel, A. Chelerythrine and sanguinarine. Arch. Pharm. 1924, 262, 488–500. [Google Scholar]
- Slavik, J. Alkaloide der mohngewachse (Papaveraceae) X. Uber die nebenalkaloide des klatschmohns (Papaver rhoeas L.) und der herzblume (Dicentra spectabilis L.). Collect. Czech. Chem. Commun. 1959, 24, 2506–2515. [Google Scholar] [CrossRef]
- Suchomelova, J.; Bochorakova, H.; Paulova, H.; Musil, P.; Taborska, E. HPLC quantification of seven quaternary benzo[c]phenanthridine alkaloids in six species of the family papaveraceae. J. Pharm. Biomed. Anal. 2007, 44, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Affolter, J.; Randle, W. Spatial and temporal distribution of the alkaloid sanguinarine in Sanguinaria canadensis L. (bloodroot). Econ. Bot. 2007, 61, 223–234. [Google Scholar] [CrossRef]
- Homerberg, V.; Beringer, G. What is the proper time for the collection of sanguinaria? J. Am. Pharm. Assoc. 1913, 2, 1302. [Google Scholar] [CrossRef]
- Farwell, O.A. The proper time to collect sanguinaria. Am. J. Pham. 1915, 87, 97–98. [Google Scholar]
- Graf, T.N.; Levine, K.E.; Andrews, M.E.; Perlmutter, J.M.; Nielsen, S.J.; Davis, J.M.; Wani, M.C.; Oberlies, N.H. Variability in the yield of benzophenanthridine alkaloids in wildcrafted vs. cultivated bloodroot (Sanguinaria canadensis L.). J. Agric. Food Chem. 2007, 55, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Rho, D.; Chauret, N.; Laberge, N.; Archambault, J. Growth characteristics of Sanguinaria canadensis L. Cell suspensions and immobilized cultures for production of benzophenanthridine alkaloids. Appl. Microbiol. Biotechnol. 1992, 36, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Becci, P.J.; Schwartz, H.; Barnes, H.H.; Southard, G.L. Short-term toxicity studies of sanguinarine and of two alkaloid extracts of Sanguinaria canadensis L. J. Toxicol. Environ. Health 1987, 20, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.A. Alkaloid-bearing plants: An ecogeographic perspective. Am. Nat. 1976, 110, 261–284. [Google Scholar] [CrossRef]
- Jeanne, R.L. A latitudinal gradient in rates of ant predation. Ecology 1979, 60, 1211–1224. [Google Scholar] [CrossRef]
- Levin, D.A. The toxicity of plant alkaloids: An ecogeographic perspective. Biochem. Syst. Ecol. 1978, 6, 61–76. [Google Scholar] [CrossRef]
- Chandra, P.; Purohit, A. Berberine contents and alkaloid profile of berberis species from different altitudes. Biochem. Syst. Ecol. 1980, 8, 379–380. [Google Scholar] [CrossRef]
- Carey, D.B.; Wink, M. Elevational variation of quinolizidine alkaloid contents in a lupine (Lupinus argenteus) of the Rocky Mountains. J. Chem. Ecol. 1994, 20, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Pencikova, K.; Urbanova, J.; Musil, P.; Taborska, E.; Gregorova, J. Seasonal variation of bioactive alkaloid contents in Macleaya microcarpa (Maxim.) Fedde. Molecules 2011, 16, 3391–3401. [Google Scholar] [CrossRef] [PubMed]
- Arnason, J.; Guerin, B.; Kraml, M.; Mehta, B.; Redmond, R.; Scaiano, J. Phototoxic and photochemical properties of sanguinarine. Photochem. Photobiol. 1992, 55, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Maiti, M.; Nandi, R.; Chaudhuri, K. The effect of PH on the absorption and fluorescence-spectra of sanguinarine. Photochem. Photobiol. 1983, 38, 245–249. [Google Scholar] [CrossRef]
- Jones, R.R.; Harkrader, R.J.; Southard, G.L. The effect of PH on sanguinarine iminium ion form. J. Nat. Prod. 1986, 49, 1109–1111. [Google Scholar] [CrossRef]
- Bajaj, N.P.; McLean, M.J.; Waring, M.J.; Smekal, E. Sequence-selective, pH-dependent binding to DNA of benzophenanthridine alkaloids. J. Mol. Recognit. 1990, 3, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Todor, I. The effect of the antineoplastic drug ukrain on the electrokinetic potential of malignant and normal cells. Int. J. Immunother. 2003, 19, 159–168. [Google Scholar]
- Maiti, M.; Das, S.; Sen, A.; Das, A.; Kumar, G.S.; Nandi, R. Influence of DNA structures on the conversion of sanguinarine alkanolamine form to iminium form. J. Biomol. Struct. Dyn. 2002, 20, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Slaninova, I.; Slanina, J.; Taborska, E. Quaternary benzo[c]phenanthridine alkaloids—Novel cell permeant and red fluorescing DNA probes. Cytom. A 2007, 71, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.P.; Zhao, Z.Z.; Cai, Z.; Jiang, Z.H. DNA-binding affinities and sequence selectivity of quaternary benzophenanthridine alkaloids sanguinarine, chelerythrine, and nitidine. Bioorg. Med. Chem. 2006, 14, 5439–5445. [Google Scholar] [CrossRef] [PubMed]
- Byrn, S.R.; Dolch, G.D. Analysis of binding of daunorubicin and doxorubicin to DNA using computerized curve-fitting procedures. J. Pharm. Sci. 1978, 67, 688–693. [Google Scholar] [CrossRef]
- Messori, L.; Temperini, C.; Piccioli, F.; Animati, F.; Di Bugno, C.; Orioli, P. Solution chemistry and DNA binding properties of men 10755, a novel disaccharide analogue of doxorubicin. Bioorg. Med. Chem. 2001, 9, 1815–1825. [Google Scholar] [CrossRef]
- Adhami, V.M.; Aziz, M.H.; Reagan-Shaw, S.R.; Nihal, M.; Mukhtar, H.; Ahmad, N. Sanguinarine causes cell cycle blockade and apoptosis of human prostate carcinoma cells via modulation of cyclin kinase inhibitor-cyclin-cyclin-dependent kinase machinery. Mol. Cancer Ther. 2004, 3, 933–940. [Google Scholar] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase ii in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Holy, J.; Lamont, G.; Perkins, E. Disruption of nucleocytoplasmic trafficking of cyclin D1 and topoisomerase II by sanguinarine. BMC Cell Biol. 2006, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Vos, S.M.; Tretter, E.M.; Schmidt, B.H.; Berger, J.M. All tangled up: How cells direct, manage and exploit topoisomerase function. Nat. Rev. Mol. Cell Biol. 2011, 12, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Okamoto, K. Structural insights into G-quadruplexes: Towards new anticancer drugs. Future Med. Chem. 2010, 2, 619–646. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.P.; Hagihara, M.; Jiang, Z.H.; Nakatani, K. Ligand binding to tandem g quadruplexes from human telomeric DNA. ChemBioChem 2008, 9, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.N.; Xie, M.X. Competitive binding assay for G-quadruplex DNA and sanguinarine based on room temperature phosphorescence of Mn-doped ZnS quantum dots. J. Photochem. Photobiol. A Chem. 2014, 279, 24–31. [Google Scholar] [CrossRef]
- Cummaro, A.; Fotticchia, I.; Franceschin, M.; Giancola, C.; Petraccone, L. Binding properties of human telomeric quadruplex multimers: A new route for drug design. Biochimie 2011, 93, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Sun, H.; Zhou, H.; Xiang, J.; Tang, Y.; Zhao, C. The interaction of telomeric DNA and c-myc22 G-quadruplex with 11 natural alkaloids. Nucleic Acid Ther. 2012, 22, 127–136. [Google Scholar] [PubMed]
- Lopus, M.; Panda, D. The benzophenanthridine alkaloid sanguinarine perturbs microtubule assembly dynamics through tubulin binding. A possible mechanism for its antiproliferative activity. FEBS J. 2006, 273, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.; Knipling, L. Antimicrotubule properties of benzophenanthridine alkaloids. Biochemistry 1993, 32, 13334–13339. [Google Scholar] [CrossRef] [PubMed]
- Kinniburgh, A.J. A cis-acting transcription element of the c-myc gene can assume an H-DNA conformation. Nucleic Acids Res. 1989, 17, 7771–7778. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.C.; Chastain, P.; Lee, J.S.; Hegde, B.G.; Houston, S.; Langen, R.; Hsieh, C.-L.; Haworth, I.S.; Lieber, M.R. Evidence for a triplex DNA conformation at the Bcl-2 major breakpoint region of the t (14; 18) translocation. J. Biol. Chem. 2005, 280, 22749–22760. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.D.; Bender, C.; Mannsperger, H.; Buergy, D.; Kambakamba, P.; Mudduluru, G.; Korf, U.; Hughes, D.; Van Dyke, M.W.; Allgayer, H. Triplex DNA-binding proteins are associated with clinical outcomes revealed by proteomic measurements in patients with colorectal cancer. Mol. Cancer 2012, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Latimer, L.J.; Payton, N.; Forsyth, G.; Lee, J.S. The binding of analogues of coralyne and related heterocyclics to DNA triplexes. Biochem. Cell Biol. 1995, 73, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kumar, G.S.; Ray, A.; Maiti, M. Spectroscopic and thermodynamic studies on the binding of sanguinarine and berberine to triple and double helical DNA and RNA structures. J. Biomol. Struct. Dyn. 2003, 20, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kumar, G.S.; Maiti, M. Conversions of the left-handed form and the protonated form of DNA back to the bound right-handed form by sanguinarine and ethidium: A comparative study. Biophys. Chem. 1999, 76, 199–218. [Google Scholar] [CrossRef]
- Rich, A. Speculation on the biological roles of left-handed Z-DNAa. Ann. N. Y. Acad. Sci. 1994, 726, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Selvi, B.R.; Pradhan, S.K.; Shandilya, J.; Das, C.; Sailaja, B.S.; Shankar, G.N.; Gadad, S.S.; Reddy, A.; Dasgupta, D.; Kundu, T.K. Sanguinarine interacts with chromatin, modulates epigenetic modifications, and transcription in the context of chromatin. Chem. Biol. 2009, 16, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.L.; Graves, D.E.; Britt, M.; Chaires, J.B. Inhibition of the B to Z transition in poly (dGdC). Cntdot. Poly (dGdC) by covalent attachment of ethidium: Kinetic studies. Biochemistry 1991, 30, 10931–10937. [Google Scholar] [CrossRef] [PubMed]
- Giri, P.; Kumar, G.S. Molecular aspects of small molecules-poly(A) interaction: An approach to RNA based drug design. Curr. Med. Chem. 2009, 16, 965–987. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Islam, M.M.; Kumar, G.S. Binding of the anticancer alkaloid sanguinarine to double stranded rnas: Insights into the structural and energetics aspects. Mol. Biosyst. 2010, 6, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Kaur, S.; George, J.; Nihal, M.; Hahn, M.C.P.; Scarlett, C.O.; Ahmad, N. Molecular signatures of sanguinarine in human pancreatic cancer cells: A large scale label-free comparative proteomics approach. Oncotarget 2015, 6, 10335–10348. [Google Scholar] [CrossRef] [PubMed]
- Matkar, S.S.; Wrischnik, L.A.; Hellmann-Blumberg, U. Production of hydrogen peroxide and redox cycling can explain how sanguinarine and chelerythrine induce rapid apoptosis. Arch. Biochem. Biophys. 2008, 477, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Amstad, P.; He, P.; Robles, A.; Lupold, S.; Kaneko, I.; Ichimiya, M.; Sengupta, S.; Mechanic, L.; Okamura, S. P53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004, 64, 2350–2356. [Google Scholar] [CrossRef] [PubMed]
- Vrba, J.; Hrbac, J.; Ulrichova, J.; Modriansky, M. Sanguinarine is a potent inhibitor of oxidative burst in DMSO-differentiated HL-60 cells by a non-redox mechanism. Chem. Biol. Interact. 2004, 147, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Slaninova, I.; Pencikova, K.; Urbanova, J.; Slanina, J.; Taborska, E. Antitumour activities of sanguinarine and related alkaloids. Phytochem. Rev. 2014, 13, 51–68. [Google Scholar] [CrossRef]
- Gu, S.; Yang, X.-C.; Xiang, X.-Y.; Wu, Y.A.O.; Zhang, Y.U.; Yan, X.-Y.; Xue, Y.-N.; Sun, L.-K.; Shao, G.-G. Sanguinarine-induced apoptosis in lung adenocarcinoma cells is dependent on reactive oxygen species production and endoplasmic reticulum stress. Oncol. Rep. 2015, 34, 913–919. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kaufman, R.J. The Endoplasmic Reticulum and the Unfolded Protein Response. Semin. Cell Dev. Biol. 2007, 18, 716–731. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.D.; Kaufman, R.J. Er stress and its functional link to mitochondria: Role in cell survival and death. Cold Spring Harb. Perspect. Biol. 2011, 3, a004424. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007, 9, 2277–2294. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Zimmerman, T.; Gomez del Pulgar, T.; Moyer, M.P.; Lacal Sanjuan, J.C.; Cebrian, A. Choline kinase inhibition induces exacerbated endoplasmic reticulum stress and triggers apoptosis via chop in cancer cells. Cell Death Dis. 2013, 4, e933. [Google Scholar] [CrossRef] [PubMed]
- Coffey, R.N.; Watson, R.W.G.; Hegarty, N.J.; O’Neill, A.; Gibbons, N.; Brady, H.R.; Fitzpatrick, J.M. Thiol-mediated apoptosis in prostate carcinoma cells. Cancer 2000, 88, 2092–2104. [Google Scholar] [CrossRef]
- Hall, A. The role of glutathione in the regulation of apoptosis. Eur. J. Clin. Investig. 1999, 29, 238–245. [Google Scholar] [CrossRef]
- Kerbel, R.S. Tumor angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.; Folkman, J. Clinical translation of angiogenesis inhibitors. Nat. Rev. Cancer 2002, 2, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.P.; Koh, G.Y. Suppression of angiogenesis by the plant alkaloid, sanguinarine. Biochem. Biophys. Res. Commun. 2004, 317, 618–624. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, I.; Raspaglio, G.; Zannoni, G.F.; Travaglia, D.; Prisco, M.G.; Mosca, M.; Ferlini, C.; Scambia, G.; Gallo, D. Antiproliferative and antiangiogenic effects of the benzophenanthridine alkaloid sanguinarine in melanoma. Biochem. Pharmacol. 2009, 78, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Malikova, J.; Zdarilova, A.; Hlobilkova, A.; Ulrichova, J. The effect of chelerythrine on cell growth, apoptosis, and cell cycle in human normal and cancer cells in comparison with sanguinarine. Cell Biol. Toxicol. 2006, 22, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Chmura, S.J.; Dolan, M.E.; Cha, A.; Mauceri, H.J.; Kufe, D.W.; Weichselbaum, R.R. In vitro and in vivo activity of protein kinase C inhibitor chelerythrine chloride induces tumor cell toxicity and growth delay in vivo. Clin. Cancer Res. 2000, 6, 737–742. [Google Scholar] [PubMed]
- Yu, R.; Mandlekar, S.; Tan, T.-H.; Kong, A.-N.T. Activation of p38 and c-Jun N-terminal kinase pathways and induction of apoptosis by chelerythrine do not require inhibition of protein kinase C. J. Biol. Chem. 2000, 275, 9612–9619. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.-L.; Lee, M.C.; Tan, K.O.; Yang, L.-K.; Lee, A.S.; Flotow, H.; Fu, N.Y.; Butler, M.S.; Soejarto, D.D.; Buss, A.D. Identification of Chelerythrine as an inhibitor of BclXL function. J. Biol. Chem. 2003, 278, 20453–20456. [Google Scholar] [CrossRef] [PubMed]
- Medvetz, D.; Priolo, C.; Henske, E.P. Therapeutic targeting of cellular metabolism in cells with hyperactive mTORC1: A paradigm shift. Mol. Cancer Res. 2015, 13, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Slaninova, I.; Slunska, Z.; Sinkora, J.; Vlkova, M.; Taborska, E. Screening of minor benzo[c]phenanthridine alkaloids for antiproliferative and apoptotic activities. Pharm. Biol. 2007, 45, 131–139. [Google Scholar] [CrossRef]
- Hammerova, J.; Uldrijan, S.; Taborska, E.; Slaninova, I. Benzo[c]phenanthridine alkaloids exhibit strong anti-proliferative activity in malignant melanoma cells regardless of their p53 status. J. Dermatol. Sci. 2011, 62, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Slunska, Z.; Gelnarova, E.; Hammerova, J.; Taborska, E.; Slaninova, I. Effect of quaternary benzo[c]phenanthridine alkaloids sanguilutine and chelilutine on normal and cancer cells. Toxicol. in Vitro 2010, 24, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Hammerova, J.; Uldrijan, S.; Taborska, E.; Vaculova, A.H.; Slaninova, I. Necroptosis modulated by autophagy is a predominant form of melanoma cell death induced by sanguilutine. Biol. Chem. 2012, 393, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Galluzzi, L.; Berghe, T.V.; Kroemer, G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Yuan, Y.; Ding, Y.Z.; Jin, T.F.; Pei, G. Study on the alkaloids from the stem of zanthoxylum dissitum. Zhong Yao Cai 2011, 34, 551–553. [Google Scholar] [PubMed]
- He, K.; Gao, J.-L. Protopine inhibits heterotypic celladhesion in MDA-MB-231 cells through down-regulation of multi-adhesive factors. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, M.M.; Kumar, A.; Darnay, B.G.; Chainy, G.B.; Agarwal, S.; Aggarwal, B.B. Sanguinarine (pseudochelerythrine) is a potent inhibitor of NF-κB activation, Iκα phosphorylation, and degradation. J. Biol. Chem. 1997, 272, 30129–30134. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, P.H.; Wan, K.F.; Sivaraman, T.; Xu, J.; Moore, F.K.; Hung, A.W.; Mok, H.Y.K.; Yu, V.C.; Chai, C.L.L. Structure-activity relationship studies of phenanthridine-based Bcl-Xl inhibitors. J. Med. Chem. 2008, 51, 6699–6710. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xiang, J.F.; Yang, Q.F.; Li, Q.A.; Zhou, Q.J.; Zhang, X.F.; Tang, Y.L.; Xu, G.Z. Formation of human telomeric G-quadruplex structures induced by the quaternary benzophenanthridine alkaloids: Sanguinarine, nitidine, and chelerythrine. Chin. J. Chem. 2010, 28, 771–780. [Google Scholar] [CrossRef]
- Ahsan, H.; Reagan-Shaw, S.; Breur, J.; Ahmad, N. Sanguinarine induces apoptosis of human pancreatic carcinoma AsPC-1 and BxPC-3 cells via modulations in Bcl-2 family proteins. Cancer Lett. 2007, 249, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Kim, S.O.; Kim, G.Y.; Kwon, T.K.; Choi, B.T.; Lee, W.H.; Choi, Y.H. Induction of apoptosis by sanguinarine in C6 rat glioblastoma cells is associated with the modulation of the Bcl-2 family and activation of caspases through downregulation of extracellular signal-regulated kinase and Akt. Anticancer Drugs 2007, 18, 913–921. [Google Scholar] [PubMed]
- Hussain, A.R.; Al-Jomah, N.A.; Siraj, A.K.; Pulicat, M.S.; Al-Hussein, K.A.; Al-Kuraya, K.S.; Uddin, S. Up-regulation of death receptor 5 and bax translocation is necessary to induce apoptosis by sanguinarine in primary effusion lymphoma. Blood 2006, 108, 234B. [Google Scholar]
- Ghosh, S.; Jana, J.; Kar, R.K.; Chatterjee, S.; Dasgupta, D. Plant alkaloid chelerythrine induced aggregation of human telomere sequence—A unique mode of association between a small molecule and a quadruplex. Biochemistry 2015, 54, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Barreto, M.C.; Pinto, R.E.; Arrabaca, J.D.; Pavao, M.L. Inhibition of mouse liver respiration by Chelidonium majus isoquinoline alkaloids. Toxicol. Lett. 2003, 146, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.G.; Rojas, C.; Tanega, C.; Shen, M.; Simeonov, A.; Boxer, M.B.; Auld, D.S.; Ferraris, D.V.; Tsukamoto, T.; Slusher, B.S. Kinetic characterization of ebselen, chelerythrine and apomorphine as glutaminase inhibitors. Biochem. Biophys. Res. Commun. 2013, 438, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Shemon, A.N.; Sluyter, R.; Conigrave, A.D.; Wiley, J.S. Chelerythrine and other benzophenanthridine alkaloids block the human P2X7 receptor. Br. J. Pharmacol. 2004, 142, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.; Lai, J.; Yong, J.; Li, S.F.Y.; Leung, T. Chelerythrine perturbs lamellar actomyosin filaments by selective inhibition of myotonic dystrophy kinase-related Cdc42-binding kinase. FEBS Lett. 2011, 585, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-F.; Guo, Y.; Zhang, J.-B.; Wei, X.-H. Induction of apoptosis by chelerythrine chloride through mitochondrial pathway and Bcl-2 family proteins in human hepatoma SMMC-7721 cell. Arch. Pharm. Res. 2011, 34, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Slaninová, I.; Táborská, E.; Bochořáková, H.; Slanina, J. Interaction of benzo[c]phenanthridine and protoberberine alkaloids with animal and yeast cells. Cell Biol. Toxicol. 2001, 17, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, J.; Hu, J.; Zhu, X.; Yang, H.; Wang, C.; Zhang, Y. Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca2+ antagonism and antioxidant mechanisms. Eur. J. Pharmacol. 2008, 591, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Schmeller, T.; Latz-Brüning, B.; Wink, M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry 1997, 44, 257–266. [Google Scholar] [CrossRef]
- Caballero-George, C.; Vanderheyden, P.M.; Apers, S.; van den Heuvel, H.; Solis, P.N.; Gupta, M.P.; Claeys, M.; Pieters, L.; Vauquelin, G.; Vlietinck, A.J. Inhibitory activity on binding of specific ligands to the human angiotensin II AT1 and endothelin 1 ETA receptors: Bioactive benzo[c]phenanthridine alkaloids from the root of Bocconia frutescens. Planta Med. 2002, 68, 770–775. [Google Scholar] [CrossRef]
- Caballero-George, C.; Vanderheyden, P.; Solis, P.; Pieters, L.; Shahat, A.; Gupta, M.; Vauquelin, G.; Vlietinck, A. Biological screening of selected medicinal panamanian plants by radioligand-binding techniques. Phytomedicine 2001, 8, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Caballero-George, C.; Vanderheyden, P.M.L.; Solis, P.N.; Gupta, M.P.; Pieters, L.; Vauquelin, G.; Vlietinck, A. In vitro effect of sanguinarine alkaloid on binding of [3H] candesartan to the human angiotensin AT1 receptor. Eur. J. Pharmacol. 2003, 458, 257–262. [Google Scholar] [CrossRef]
- Li, B.; Wu, Q.; Shi, J.-S.; Sun, A.-S.; Huang, X.-N. Effects of protopine on intracellular calcium and the PKC activity of rat aorta smooth muscle. Sheng Li Xue Bao 2005, 57, 240–246. [Google Scholar] [PubMed]
- Seifen, E.; Adams, R.J.; Riemer, R.K. Sanguinarine: A positive inotropic alkaloid which inhibits cardiac Na+,K+-ATPase. Eur. J. Pharmacol. 1979, 60, 373–377. [Google Scholar] [CrossRef]
- Song, L.S.; Ren, G.J.; Chen, Z.L.; Chen, Z.H.; Zhou, Z.N.; Cheng, H. Electrophysiological effects of protopine in cardiac myocytes: Inhibition of multiple cation channel currents. Br. J. Pharmacol. 2000, 129, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Liu, Y.; Li, Z.; Yang, X.; Wang, H.; Wen, Y.; Chen, Y. Effect of α-allocryptopine on transient outward potassium current in rabbit ventricular myocytes. Cardiology 2008, 111, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Akbarov, Z.S.; Aliev, K.; Sultanov, M.B. Comparative study of the anti-arrhythmic action of the alkaloid a-allocryptopine with quinidine. Dokl. Akad. Nauk Uzb. 1972, 29, 38. [Google Scholar]
- Yamamoto, S.; Seta, K.; Morisco, C.; Vatner, S.F.; Sadoshima, J. Chelerythrine rapidly induces apoptosis through generation of reactive oxygen species in Cardiac myocytes. J. Mol. Cell. Cardiol. 2001, 33, 1829–1848. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.S.; Kim, Y.H.; Pan, C.-H.; Nho, C.W.; Samdan, J.; Yansan, J.; Lee, J.K. Protopine reduces the inflammatory activity of lipopolysaccharide-stimulated murine macrophages. BMB Rep. 2012, 45, 108. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.-F.; Zhou, P.; Li, W.-F.; Xu, H.-B. Effects of chelerythrine, a specific inhibitor of cyclooxygenase-2, on acute inflammation in mice. Fitoterapia 2011, 82, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Vavreckova, C.; Gawlik, I.; Muller, K. Benzophenanthridine alkaloids of chelidonium majus; I. Inhibition of 5- and 12-lipoxygenase by a non-redox mechanism. Planta Med. 1996, 62, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Siomboing, X.; Gressier, B.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.-C. Investigation of the inhibitory effects of chelerythrine chloride on the translocation of the protein kinase c βi, βii, ζ in human neutrophils. IL Farmaco 2001, 56, 859–865. [Google Scholar] [CrossRef]
- Vrba, J.; Dvorak, Z.; Ulrichova, J.; Modriansky, M. Conventional protein kinase c isoenzymes undergo dephosphorylation in neutrophil-like HL-60 cells treated by chelerythrine or sanguinarine. Cell Biol. Toxicol. 2008, 24, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.; Meagher, L.C.; MacDermot, J.; Haslett, C. Impairment of function in aging neutrophils is associated with apoptosis. J. Immunol. 1993, 150, 5124–5134. [Google Scholar] [PubMed]
- Haslett, C. Resolution of acute inflammation and the role of apoptosis in the tissue fate of granulocytes. Clin. Sci. 1992, 83, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Grigg, J.; Silverman, M.; Savill, J.; Sarraf, C.; Haslett, C. Neutrophil apoptosis and clearance from neonatal lungs. Lancet 1991, 338, 720–722. [Google Scholar] [CrossRef]
- Savill, J.; Smith, J.; Sarraf, C.; Ren, Y.; Abbott, F.; Rees, A. Glomerular mesangial cells and inflammatory macrophages ingest neutrophils undergoing apoptosis. Kidney Int. 1992, 42, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.F.; Nguyen, P.K.; Atkins, K.B.; Hinshaw, D.B. Chelerythrine chloride induces rapid polymorphonuclear leukocyte apoptosis through activation of caspase-3. Shock 2000, 13, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Albelda, S.M.; Smith, C.W.; Ward, P. Adhesion molecules and inflammatory injury. FASEB J. 1994, 8, 504–512. [Google Scholar] [PubMed]
- Tang, M.; Fiscus, L. Important roles for l-selectin and icam-1 in the development of allergic airway inflammation in asthma. Pulm. Pharmacol. Ther. 2001, 14, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Malik, P.; Arora, S.K.; Mukherjee, T.K. Intercellular adhesion molecule-1 as a drug target in asthma and rhinitis. Respirology 2014, 19, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Luo, S.F.; Hsieh, H.L.; Chi, P.L.; Lin, C.C.; Wu, C.C.; Hsiao, L.D. Interleukin-1β induces ICAM-1 expression enhancing leukocyte adhesion in human rheumatoid arthritis synovial fibroblasts: Involvement of erk, JNK, AP-1, and NF-κB. J. Cell. Physiol. 2010, 224, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Van Kuijk, A.W.; Reinders-Blankert, P.; Smeets, T.J.; Dijkmans, B.A.; Tak, P.P. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: Implications for treatment. Ann. Rheum. Dis. 2006, 65, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Sakata, Y.; Morimoto, K.; Tambe, Y.; Watanabe, Y.; Honda, G.; Tabata, M.; Oshima, T.; Masuda, T.; Umezawa, T. Influence of natural and synthetic compounds on cell surface expression of cell adhesion molecules, ICAM-1 and VCAM-1. Planta Med. 2001, 67, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Obiang-Obounou, B.W.; Kang, O.H.; Choi, J.G.; Keum, J.H.; Kim, S.B.; Mun, S.H.; Shin, D.W.; Kim, K.W.; Park, C.B.; Kim, Y.G.; et al. The mechanism of action of sanguinarine against methicillin-resistant staphylococcus aureus. J. Toxicol. Sci. 2011, 36, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hamoud, R.; Reichling, J.; Wink, M. Synergistic antimicrobial activity of combinations of sanguinarine and edta with vancomycin against multidrug resistant bacteria. Drug Metab. Lett. 2014, 8, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Dzink, J.L.; Socransky, S.S. Comparative in vitro activity of sanguinarine against oral microbial isolates. Antimicrob. Agents Chemother. 1985, 27, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Fischbach, L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010, 59, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B.; Pendland, S.L.; Stoia, A.; Chadwick, L.R. In vitro susceptibility of Helicobacter pylori to isoquinoline alkaloids from Sanguinaria canadensis and Hydrastis canadensis. Phytother. Res. 2003, 17, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Newton, S.M.; Lau, C.; Gurcha, S.S.; Besra, G.S.; Wright, C.W. The evaluation of forty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J. Ethnopharmacol. 2002, 79, 57–67. [Google Scholar] [CrossRef]
- Seubert, J.; Pohlke, R.; Loebich, F. Synthesis and properties of praziquantel, a novel broad spectrum anthelmintic with excellent activity against schistosomes and cestodes. Experientia 1977, 33, 1036–1037. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Botros, S.; Metwally, A.; William, S.; Farghally, A.; Tao, L.-F.; Day, T.A.; Bennett, J.L. Resistance to praziquantel: Direct evidence from schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg. 1999, 60, 932–935. [Google Scholar] [PubMed]
- Zhang, S.-M.; Coultas, K.A. Identification of plumbagin and sanguinarine as effective chemotherapeutic agents for treatment of schistosomiasis. Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Schär, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides stercoralis: Global distribution and risk factors. PLoS Negl. Trop. Dis. 2013, 7, e2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segarra-Newnham, M. Manifestations, diagnosis, and treatment of strongyloides stercoralis infection. Ann. Pharmacother. 2007, 41, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Koga, M.; Matsuhashi, R.; Koike, K.; Tada, I.; Nikaido, T. Assay of nematocidal activity of isoquinoline alkaloids using third-stage larvae of Strongyloides ratti and S. Venezuelensis. Vet. Parasitol. 2002, 104, 131–138. [Google Scholar] [CrossRef]
- Kaewviyudth, S.; Chinabut, S. Five new species of Dactylogyrus (Monogenea) from cyprimd fishes in Thailand. Asian Fish. Sci. 1999, 12, 391–399. [Google Scholar]
- Wang, G.X.; Zhou, Z.; Jiang, D.X.; Han, J.; Wang, J.F.; Zhao, L.W.; Li, J. In vivo anthelmintic activity of five alkaloids from Macleaya microcarpa (Maxim.) fedde against Dactylogyrus intermedius in Carassius auratus. Vet. Parasitol. 2010, 171, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Akao, N.; Matsuhashi, R.; Koike, K.; Fujita, K.; Nikaido, T. Inhibitory effect of isoquinoline alkaloids on movement of second-stage larvae of Toxocara canis. Biol. Pharm. Bull. 2002, 25, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.J.; Goodsell, D.S.; Kan, C.C. Identification of sanguinarine as a novel HIV protease inhibitor from high-throughput screening of 2000 drugs and natural products with a cell-based assay. Lett. Drug Des. Discov. 2005, 2, 364–371. [Google Scholar] [CrossRef]
- Orhan, I.; Özcelik, B.; Şener, B. Antiviral and Antimicrobial Evaluation of Some Heterocyclic Compounds from Turkish Plants; Springer: Berlin, Germany, 2007; Volume 11, pp. 303–323. [Google Scholar]
- Wangchuk, P.; Bremner, J.B.; Rattanajak, R.; Kamchonwongpaisan, S. Antiplasmodial agents from the bhutanese medicinal plant Corydalis calliantha. Phytother. Res. 2010, 24, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, V.; Wink, M. Alkaloids induce programmed cell death in bloodstream forms of trypanosomes (Trypanosoma b. brucei). Molecules 2008, 13, 2462–2473. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zuo, G.; Hao, X.; Wang, G.; Xiao, H.; Zhang, J.; Xu, G. Antifungal activity of the benzo[c]phenanthridine alkaloids from Chelidonium majus Linn against resistant clinical yeast isolates. J. Ethnopharmacol. 2009, 125, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Pong Ng, Y.; Tsun Or, T.C.; Ip, N.Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int. 2015, 89, 260–270. [Google Scholar]
- Kim, S.R.; Hwang, S.Y.; Jang, Y.P.; Park, M.J.; Markelonis, G.J.; Oh, T.H.; Kim, Y.C. Protopine from corydalis ternata has anticholinesterase and antiamnesic activities. Planta Med. 1999, 65, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lee, K.T.; Baek, N.-I.; Kim, S.-H.; Park, H.W.; Lim, J.P.; Shin, T.Y.; Eom, D.O.; Yang, J.H.; Eun, J.S. Acetylcholinesterase inhibitors from the aerial parts of Corydalis speciosa. Arch. Pharm. Res. 2004, 27, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Şener, B.; Orhan, İ. Discovery of drug candidates from some turkish plants and conservation of biodiversity. Pure Appl. Chem. 2005, 77, 53–64. [Google Scholar] [CrossRef]
- Zheng, W.; Qiu, L.; Wang, R.; Feng, X.; Han, Y.; Zhu, Y.; Chen, D.; Liu, Y.; Jin, L.; Li, Y. Selective targeting of PPARΓ by the natural product chelerythrine with a unique binding mode and improved antidiabetic potency. Sci. Rep. 2015, 5, 12222. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Huh, J.; Choi, M.; Yoon, S.Y.; Yang, S.; Hong, H.; Cho, S. Regulation of glutamate level in rat brain through activation of glutamate dehydrogenase by Corydalis ternata. Exp. Mol. Med. 2005, 37, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-F.; Chu, W.-J.; Qing, X.-Y.; Li, S.; Wang, X.-S.; Qing, G.-W.; Fei, J.; Guo, L.-H. Protopine inhibits serotonin transporter and noradrenaline transporter and has the antidepressant-like effect in mice models. Neuropharmacology 2006, 50, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Engel, R. Ueber Das Protopin. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1890, 27, 419–431. [Google Scholar] [CrossRef]
- Goto, K.; Oda, R. Studies on the Alkaloids of Macleya cordata R. J. Pharm. Soc. Jpn. 1949, 69, 307. [Google Scholar]
- Abu-Ghalyun, Y.; Masalmeh, A.; Al-Khalil, S. Effects of allocryptopine, an alkaloid isolated from glaucium arabicum on rat isolated ileum and urinary bladder. Gen. Pharmacol. Vasc. Syst. 1997, 29, 621–623. [Google Scholar] [CrossRef]
- Üstünes, L.; Laekeman, G.M.; Gözler, B.; Vlietinck, A.J.; Özer, A.; Herman, A.G. In vitro study of the anticholinergic and antihistaminic activities of protopine and some derivatives. J. Nat. Prod. 1988, 51, 1021–1022. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.H.; Wu, H.L.; Lin, B.R.; Lan, W.H.; Chang, H.H.; Ho, Y.S.; Lee, P.H.; Wang, Y.J.; Wang, J.S.; Chen, Y.J.; et al. Antiplatelet effect of sanguinarine is correlated to calcium mobilization, thromboxane and camp production. Atherosclerosis 2007, 191, 250–258. [Google Scholar] [CrossRef] [PubMed]
- European Union. Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect (1831/2003/ec); European Commission: Brussels, Belgium, 2005. [Google Scholar]
- Roth, R.; Kirchgessner, M. Organic acids as feed additives for young pigs: Nutritional and gastrointestinal effects. J. Anim. Feed Sci. 1998, 7, 25–33. [Google Scholar]
- Blank, R.; Muller-Siegwardt, B.; Wolffram, S. Sanguinarine does not influence availability or metabolism of tryptophan in pigs. Livest. Sci. 2010, 134, 24–26. [Google Scholar] [CrossRef]
- Stiborova, M.; Vostalova, J.; Zdarilova, A.; Ulrichova, J.; Hudecek, J.; Tschirner, K.; Simanek, V. Macleaya cordata extract and sangrovit genotoxicity. Assessment in vivo. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov. 2008, 152, 35–39. [Google Scholar] [CrossRef]
- Kantas, D.; Papatsiros, V.G.; Tassis, P.D.; Athanasiou, L.V.; Tzika, E.D. The effect of a natural feed additive (Macleaya cordata), containing sanguinarine, on the performance and health status of weaning pigs. Anim. Sci. J. 2015, 86, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.; Oyarzabal, O.; Freitas, D.; Berres, J.; Pena, J.; Torres, C.; Coneglian, J. Performance of broilers fed diets supplemented with sanguinarine-like alkaloids and organic acids. J. Appl. Poult. Res. 2008, 17, 128–133. [Google Scholar] [CrossRef]
- Rawling, M.D.; Merrifield, D.L.; Davies, S.J. Preliminary assessment of dietary supplementation of sangrovit® on red tilapia (Oreochromis niloticus) growth performance and health. Aquaculture 2009, 294, 118–122. [Google Scholar] [CrossRef]
- Pickler, L.; Beirão, B.C.; Hayashi, R.M.; Durau, J.F.; Lourenço, M.C.; Caron, L.F.; Santin, E. Effect of sanguinarine in drinking water on salmonella control and the expression of immune cells in peripheral blood and intestinal mucosa of broilers. J. Appl. Poult. Res. 2013, 22, 430–438. [Google Scholar] [CrossRef]
- Robbins, R.C.; Artuso-Ponte, V.C.; Moeser, A.J.; Morrow, W.E.M.; Spears, J.W.; Gebreyes, W.A. Effects of quaternary benzo[c]phenanthridine alkaloids on growth performance, shedding of organisms, and gastrointestinal tract integrity in pigs inoculated with multidrug-resistant Salmonella spp. Am. J. Vet. Res. 2013, 74, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Haldorson, G. A review of equine sarcoid. Equine Vet. Educ. 2013, 25, 210–216. [Google Scholar] [CrossRef]
- Nogueira, S.A.; Torres, S.M.; Malone, E.D.; Diaz, S.F.; Jessen, C.; Gilbert, S. Efficacy of imiquimod 5% cream in the treatment of equine sarcoids: A pilot study. Vet. Dermatol. 2006, 17, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, C. Utvärtes Behandling av Sarkoider på häst Med Aldara tm Eller Xxterra tm. slu. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 1 February 2008. [Google Scholar]
- Wilford, S.; Woodward, E.; Dunkel, B. Owners’ perception of the efficacy of newmarket bloodroot ointment in treating equine sarcoids. Can. Vet. J. 2014, 55, 683–686. [Google Scholar] [PubMed]
- Childress, M.O.; Burgess, R.C.; Holland, C.H.; Gelb, H.R. Consequences of intratumoral injection of a herbal preparation containing blood root (Sanguinaria canadensis) extract in two dogs. J. Am. Vet. Med. Assoc. 2011, 239, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, K.; Kaivosoja, S.; Seppa, S.; Knuuttila, M. Effects of sanguinaria extract on leucocytes and fibroblasts. Proc. Finn.Dent. Soc. 1988, 84, 161–165. [Google Scholar] [PubMed]
- Damm, D.D.; Curran, A.; White, D.K.; Drummond, J.E. Leukoplakia of the maxillary vestibule—An association with viadent? Oral Surg. Oral Med. 1999, 87, 61–66. [Google Scholar] [CrossRef]
- Weatherell, J.A.; Robinson, C.; Rathbone, M. The flow of saliva and its influence on the movement, deposition and removal of drugs administered to the oral cavity. Drugs Pharm. Sci. 1996, 74, 157–189. [Google Scholar]
- Munro, I.C.; Delzell, E.S.; Nestmann, E.R.; Lynch, B.S. Viadent usage and oral leukoplakia: A spurious association. Regul. Toxicol. Pharmacol. 1999, 30, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Eversole, L.R.; Eversole, G.M.; Kopcik, J. Sanguinaria-associated oral leukoplakia: Comparison with other benign and dysplastic leukoplakic lesions. Oral Surg. Oral Med. 2000, 89, 455–464. [Google Scholar] [CrossRef]
- Allen, C.L.; Loudon, J.; Mascarenhas, A.K. Sanguinaria-related leukoplakia: Epidemiologic and clinicopathologic features of a recently described entity. Gen. Dent. 2001, 49, 608–614. [Google Scholar] [PubMed]
- Eley, B.M. Antibacterial agents in the control of supragingival plaque—A review. Br. Dent. J. 1999, 186, 286–296. [Google Scholar] [PubMed]
- Jacobs, J.; Herman, P.; Heron, K.; Olsen, S.; Vaughters, L. Homeopathy for menopausal symptoms in breast cancer survivors: A preliminary randomized controlled trial. J. Altern. Complement. Med. 2005, 11, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, S.; Russell, N.; Ernst, E. Efficacy of homeopathic therapy in cancer treatment. Eur. J. Cancer 2006, 42, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Bordet, M.F.; Colas, A.; Marijnen, P.; Masson, J.L.; Trichard, M. Treating hot flushes in menopausal women with homeopathic treatment—Results of an observational study. Homeopathy 2008, 97, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Hielm-Björkman, A.; Tulamo, R.-M.; Salonen, H.; Raekallio, M. Evaluating complementary therapies for canine osteoarthritis—Part II: A homeopathic combination preparation (zeel®). Evid. Based Complement. Altern. Med. 2009, 6, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Nahler, G.; Metelmann, H.; Sperber, H. Behandlung der gonarthrose mit zeel [r] comp.-ergebnisse einer randomisierten, kontrollierten klinischen pruefung im vergleich zu hyaluronsaeure. Orthop. Pract. 1996, 32, 354–359. [Google Scholar]
- Oral Treatment of Arthritis of the Knee with Zeel® Comp. Available online: http://www.homotoxicology.net/Documents/Brochures/Zeel.pdf (accessed on 16 August 2016).
- Birnesser, H.; Klein, P.; Weiser, M. A modern homeopathic medication works as well as COX 2 inhibitors. Der Allg. 2003, 25, 261–264. [Google Scholar]
- Mohs, F.; Guyer, M. Pre-excisional fixation of tissues in the treatment of cancer in rats. Cancer Res. 1941, 1, 49–51. [Google Scholar]
- Kimyai-Asadi, A.; Goldberg, L.H.; Jih, M.H. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J. Am. Acad. Dermatol. 2005, 53, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Trost, L.B.; Bailin, P.L. History of mohs surgery. Dermatol. Clin. 2011, 29, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Mohs, F.E. Chemosurgery: A microscopically controlled method of cancer excision. Arch. Surg. 1941, 42, 279–295. [Google Scholar] [CrossRef]
- Mohs, F.E.; Caruso, R. Chemosurgery and skin cancer. AORN J. 1971, 13, 89–97. [Google Scholar] [CrossRef]
- Mohs, F.E. Chemosurgery: Microscopically controlled surgery for skin cancer—Past, present and future. J. Dermatol. Surg. Oncol. 1978, 4, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Mohs, F.E. Chemosurgery for skin cancer: Fixed tissue and fresh tissue techniques. Arch. Dermatol. 1976, 112, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Farrow, R.T. Odyssey of an American cancer specialist of a hundred years ago. Bull. Hist. Med. 1949, 23, 236–262. [Google Scholar]
- Middlesex Hospital. Report of the Surgical Staff of the Middlesex Hospital to the Weekly Board of Governors upon the Treatment of Cancerous Diseases in the Hospital on the Plan Introduced by dr. Fell; John Churchill.: London, UK, 1857. [Google Scholar]
- Journal of the American Medical Association. Bureau of investigation, comment on court opinion that internal cancer can be cured with medicine. JAMA 1951, 145, 252–253. [Google Scholar]
- Hoxsey, H.M. You Don’t Have to Die; Milestone Books: New York, NY, USA, 1956. [Google Scholar]
- Austin, S.; Baumgartner, E.; DeKadt, S. Long term follow-up of cancer patients using contreras, hoxsey and gerson therapies. J. Naturop. Med. 1995, 5, 74–76. [Google Scholar]
- Richardson, M.A.; Russell, N.C.; Sanders, T.; Barrett, R.; Salveson, C. Assessment of outcomes at alternative medicine cancer clinics: A feasibility study. J. Altern. Complement. Med. 2001, 7, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Jellinek, N.; Maloney, M.E. Escharotic and other botanical agents for the treatment of skin cancer: A review. J. Am. Acad. Dermatol. 2005, 53, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Cansema & Escharotics FAQ 200 Constituents; FAQ 206 What are the Side Effects; FAQ 215 Can it Be Used to Treat Melanoma; FAQ 222 Success Rate with Skin Cancer. Available online: http://www.altcancer.com/faqcan.htm (accessed on 15 July 2016).
- Earth Circle Creation. Available online: http://shop.earthcirclecreations.com/product_info.php?cPath=61&products_id=263 (accessed on 15 July 2016).
- Ong, N.C.; Sham, E.; Adams, B.M. Use of unlicensed black salve for cutaneous malignancy. Med. J. Aust. 2014, 200, 314. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, S.; Goldman, G.D. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch. Dermatol. 2002, 138, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Cienki, J.J.; Zaret, L. An internet misadventure: Bloodroot salve toxicity. J. Altern. Complement. Med. 2010, 16, 1125–1127. [Google Scholar] [CrossRef] [PubMed]
- Sivyer, G.W.; Rosendahl, C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: A case study. Dermatol. Pract. Concept. 2014, 4, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Osswald, S.S.; Elston, D.M.; Farley, M.F.; Alberti, J.G.; Cordero, S.C.; Kalasinsky, V.F. Self-treatment of a basal cell carcinoma with “black and yellow salve”. J. Am. Acad. Dermatol. 2005, 53, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L.; Mason, J.S.; Overington, J.P. Can we rationally design promiscuous drugs? Curr. Opin. Struct. Biol. 2006, 16, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Gupta, S.; Husain, M.M.; Heiskanen, K.M.; Mukhtar, H. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin. Cancer Res. 2000, 6, 1524–1528. [Google Scholar] [PubMed]
- Krstin, S.; Peixoto, H.S.; Wink, M. Combinations of alkaloids affecting different molecular targets with the saponin digitonin can synergistically enhance trypanocidal activity against Trypanosoma brucei. Antimicrob. Agents Chemother. 2015, 59, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Kulp, M.; Bragina, O. Capillary electrophoretic study of the synergistic biological effects of alkaloids from Chelidonium majus l. In normal and cancer cells. Anal. Bioanal. Chem. 2013, 405, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, B.J.; Tabor, M.P.; Kummer, J.A.; Leemans, C.R.; Brakenhoff, R.H. A genetic explanation of slaughter’s concept of field cancerization evidence and clinical implications. Cancer Res. 2003, 63, 1727–1730. [Google Scholar] [PubMed]
- Ansari, K.M.; Das, M. Potentiation of tumour promotion by topical application of argemone oil/isolated sanguinarine alkaloid in a model of mouse skin carcinogenesis. Chem. Biol. Interact. 2010, 188, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Reagan-Shaw, S.; Eggert, D.M.; Tan, T.C.; Afaq, F.; Mukhtar, H.; Ahmad, N. Protective effect of sanguinarine on ultraviolet B-mediated damages in SKH-1 hairless mouse skin: Implications for prevention of skin cancer. Photochem. Photobiol. 2007, 83, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Breur, J.; Ahmad, N. Enhancement of UVB radiation–mediated apoptosis by sanguinarine in hacat human immortalized keratinocytes. Mol. Cancer Ther. 2006, 5, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Gesler, W.M. Therapeutic landscapes: Medical issues in light of the new cultural geography. Soc. Sci. Med. 1992, 34, 735–746. [Google Scholar] [CrossRef]
- Harris, P.; Cooper, K.; Relton, C.; Thomas, K. Prevalence of complementary and alternative medicine (CAM) use by the general population: A systematic review and update. Int. J. Clin. Pract. 2012, 66, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Bent, S. Herbal medicine in the united states: Review of efficacy, safety, and regulation. J. Gen. Intern. Med. 2008, 23, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Board, S. Botanical medicines—The need for new regulations. N. Engl. J. Med. 2002, 347, 2073–2076. [Google Scholar]
- Coghlan, M.L.; Maker, G.; Crighton, E.; Haile, J.; Murray, D.C.; White, N.E.; Byard, R.W.; Bellgard, M.I.; Mullaney, I.; Trengove, R. Combined DNA, toxicological and heavy metal analyses provides an auditing toolkit to improve pharmacovigilance of traditional Chinese medicine (TCM). Sci. Rep. 2015, 5, 17475. [Google Scholar] [CrossRef] [PubMed]



| Alkaloids (IC50, μg/mL) | HL-60 | HeLa | KF-II | A431 |
|---|---|---|---|---|
| Sanguilutine | 0.04 | 0.46 | – | – |
| Sanguirubine | 0.12 | 0.68 | 0.22 | 0.7 |
| Chelilutine | 0.16 | 0.84 | – | – |
| Chelirubine | 0.1 | 0.37 | 0.2 | 0.28 |
| Sanguinarine | 0.34 | 0.7 | 0.5 | 0.7 |
| Chelerythrine | 0.17 | 0.48 | 0.58 | 1.44 |
| Molecular Target | Cellular Effect/Significance | Ref. |
|---|---|---|
| Sanguinarine | ||
| Topoisomerase II | Prevents DNA break repair | [114] |
| Telomere Capping | Induces rapid apoptosis | [164] |
| Oncogenes C-myc, KRAS, C-kit | Expressed in various tumors | [120] |
| H-DNA | Haematological and colorectal tumor expression | [127] |
| Bcl-2 family | Apoptosis induction | [165] |
| ERKs | Apoptosis induction | [166] |
| NF-κB | Role in proliferation, migration, apoptosis | [162] |
| DR-5 | TRAIL mediated apoptosis | [167] |
| Endoplasmic Reticulum | Unfolded Protein Response | [139] |
| VEGF-A | Impairs tumor neovascularization | [148] |
| Glutathione | Depletion amplifies oxidative stress | [24] |
| Anti-microtubule | Inhibits cell proliferation | [121] |
| Chelerythrine | ||
| Bcl-XL and Bcl-2 | Apoptosis Induction | [153] |
| Telomere Capping | Induces rapid apoptosis | [168] |
| Succinate | Cytochrome c release | [169] |
| NADH dehydrogenase | Apoptosome assembly | [169] |
| Glutaminase | Blocks Tumor Glutamine use for energy | [170] |
| mTOR | Overexpressed in Melanoma | [154] |
| Phospholipase D | Associated with angiogenesis/metastasis | [171] |
| MRCK | Impairs tumor migration | [172] |
| Tubulin Polymerization | Impairs cell division | [122] |
| MAPK | Activation results in apoptosis | [173] |
| Minor QBAs | ||
| RIP1 | Ripoptosome Formation | [158] |
| Unknown | Apoptosis ROS independent | [156] |
| Anti-microtubule | Impaired mitosis | [174] |
| Protopin Alkaloids | ||
| Antioxidant effect | May antagonize the cytotoxic effects of other alkaloids | [175] |
| EGFR/ICAM-1 | Reduced expression impairs metastasis | [161] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croaker, A.; King, G.J.; Pyne, J.H.; Anoopkumar-Dukie, S.; Liu, L. Sanguinaria canadensis: Traditional Medicine, Phytochemical Composition, Biological Activities and Current Uses. Int. J. Mol. Sci. 2016, 17, 1414. https://doi.org/10.3390/ijms17091414
Croaker A, King GJ, Pyne JH, Anoopkumar-Dukie S, Liu L. Sanguinaria canadensis: Traditional Medicine, Phytochemical Composition, Biological Activities and Current Uses. International Journal of Molecular Sciences. 2016; 17(9):1414. https://doi.org/10.3390/ijms17091414
Chicago/Turabian StyleCroaker, Andrew, Graham J. King, John H. Pyne, Shailendra Anoopkumar-Dukie, and Lei Liu. 2016. "Sanguinaria canadensis: Traditional Medicine, Phytochemical Composition, Biological Activities and Current Uses" International Journal of Molecular Sciences 17, no. 9: 1414. https://doi.org/10.3390/ijms17091414
APA StyleCroaker, A., King, G. J., Pyne, J. H., Anoopkumar-Dukie, S., & Liu, L. (2016). Sanguinaria canadensis: Traditional Medicine, Phytochemical Composition, Biological Activities and Current Uses. International Journal of Molecular Sciences, 17(9), 1414. https://doi.org/10.3390/ijms17091414