Effect of Novel Compound LX519290, a Derivative of l-allo Threonine, on Antioxidant Potential in Vitro and in Vivo
Abstract
:1. Introduction
2. Results
2.1. Radical-Scavenging Effects of LX519290 in Vitro
2.2. Cell Viability of LX519290
2.3. Inhibition Effects of LX519290 on Cellular Nitric Oxide (NO) and Reactive Oxygen Species (ROS) Generation in RAW264.7 Cells
2.4. Effects of LX519290 Against Pro-Oxidant Challenge in RAW264.7 Cells
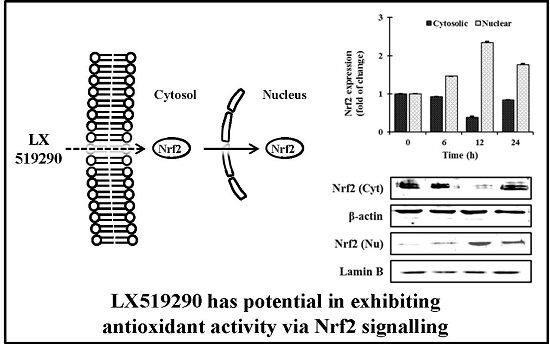
2.5. Effects of LX519290 on Gene Expressions of Antioxidants and Phase II Antioxidant Enzymes
2.6. In Vivo Antioxidant Activity of LX519290
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Care
4.3. Radical-Scavenging Activity Assays
4.4. Oxygen Radical Absorbance Capacity Assay
4.5. Cell Viability
4.6. Measurement of Cellular NO Generation
4.7. Measurement of Intracellular ROS Generation
4.8. Activity of LX519290 Against Pro-Oxidant Challenge
4.9. Total Thiol and Glutathione S-Transferase Measurement
4.10. AAPH-Induced Oxidative Stress in Rats
4.11. Reverse Transcription PCR Analysis of Gene Expression
4.12. Statistics
Author Contributions
Conflicts of Interest
References
- Imlay, J.A. Cellular defences against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008, 77, 755–776. [Google Scholar] [CrossRef] [PubMed]
- Belenky, P.; Collins, J.J. Antioxidant strategies to tolerate antibiotics. Science 2011, 334, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Jang, H.D. Nrf-2 mediated HO-1 induction coupled with the ERK signaling pathway contributes to indirect antioxidant capacity of caffeic acid phenethyl ester in HepG2 cells. Int. J. Mol. Sci. 2014, 15, 12149–12165. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-kostova, A.T.; Cheah, J.; Samouilov, A.; Zweiser, J.L.; Bosak, R.E.; Hicks, R.J.; Talalay, P. Phenolic Michael reaction acceptors: Compbined direct and indirect antioxidant defense against electrophiles and oxidants. Med. Chem. 2007, 3, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.C.; Son, H.U.; Kim, S.L.; Lee, S.H. A derivative of l-allo threonine alleviates 2,4-dinitrofluorobenzene-induced atopic dermatitis indications. Biosci. Biotechnol. Biochem. 2012, 76, 2021–2025. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.C.; Lee, S.H. Alleviation of asthma-related symptoms by a derivative of l-allo threonine. Int. J. Mol. Med. 2013, 31, 881–887. [Google Scholar] [PubMed]
- Nakajima, M.; Inukai, M.; Haneishi, T.; Terahara, A.; Arai, M. Globomycin, a new peptide antibiotic with spheroplast-forming activity. III. Structural determination of globomycin. J. Antibiot. 1978, 31, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Makart, S.; Bechtold, M.; Panke, S. Towards preparative asymmetric synthesis of β-hydroxy-α-amino acids: l-allo-threonine formation from glycine and acetaldehyde using recombinant GlyA. J. Biotechnol. 2007, 130, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.H.; Webel, D.M.; Fernandez, S.R. d-Allo threonine has no growth promoting efficacy for chicks. Poult. Sci. 1998, 77, 1397–1399. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Mazzini, F.; Bamonti, L.; Gille, L.; Bohmdorfer, S.; Piroddi, M.; Netscher, T.; Kelly, F.J.; Rosenau, T. Tocotrienamines and tocopheramines: Reactions with radials and metal ions. Bioorg. Med. Chem. 2011, 19, 6483–6491. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E.; Altun, M. Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic. Res. 2005, 39, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Schroecksnadel, S.; Gostner, J.; Zaknun, C.; Schennach, H.; Uberall, F.; Fuchs, D. Comparison of in vitro tests for antioxidant and immunomodulatory capacities of compounds. Phytomedicine 2014, 21, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant activity relationships of flavonoids and phenolic acids. Free Rad. Biol. Med 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Birari, R.; Karmase, A.; Jagtap, S.; Bhutani, K.K. Antioxidant activity of a new phenolic glycoside from Lagenaria siceraria Stand. fruits. Food Chem. 2012, 132, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Chung, W.Y.; Strain, J.J. Antioxidant (reducing) efficiency of ascorbate in plasma is not affected by concentration. J. Nutr. Biochem. 1999, 10, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Zhong, Z.; Xing, R.; Li, P.; Mo, G. The preparation and antioxidant activity of 2-[phenylhydrazine (or hydrazine)-thiosemicarbazone]-chitosan. Int. J. Biol. Macromol. 2010, 47, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.C.; Gomes, K.M.; Ferreira, J.C. Impact of exercise training on redox signaling in cardiovascular diseases. Food Chem. Toxicol. 2013, 62, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Detection of peroxyl and alkoxyl radicals produced by reaction of hydroperoxides with rat liver microsomal fractions. Biochem. J. 1989, 257, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Thompson, H.S. Antioxidants: What role do they play in physical activity and health? Am. J. Clin. Nutr. 2000, 72, 637S–646S. [Google Scholar] [PubMed]
- Rota, C.; Chignell, C.F.; Mason, R.P. Evidence for free radical formation during the oxidation of 2′,7-dichlorofluorescin to the fluorescent dye 2′,7-dichlorofluorescin by horseradish peroxidase: Possible implications for oxidative stress measurements. Free Radic. Biol. Med. 1999, 27, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Bonini, M.G.; Rota, C.; Tomasi, A.; Mason, R.P. The oxidation of 2′,7-dichlorofluorescin to reactive oxygen species: A self-fulfilling prophesy? Free Radic. Biol. Med. 2006, 40, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Ruas, C.B.; Carvalho, C.S.; Araújo, H.S.; Espíndola, E.L.; Fernandes, M.N. Oxidative stress biomarkers of exposure in the blood of cichlid species from a metal-contaminated river. Ecotoxicol. Environ. Saf. 2008, 71, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; Commodi, M.; Piroddi, L.; Incipini, L.; Sancineto, C.; Galli, S.F. Glutathione S-transferase pi expression regulates the Nrf2-dependent response to hermetic diselenides. Free Radic. Biol. Med. 2015, 88, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Kinnula, V.L.; Paakko, P.; Soini, Y. Antioxidant enzymes and redox regulating thiol proteins in malignancies of human lung. FEBS Lett. 2004, 569, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sinha, D.; Saha, P.P.; Marthala, H.; D’Silva, P. Magmas functions as a ROS regulator and provides cytoprotection against oxidative stress-mediated damages. Cell Death Dis. 2014, 5, e1394. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Gao, J.; Zheng, Y.; Wang, X.; Chen, C.; Cao, K.; Xu, J.; Li, Y.; Lu, W.; Lie, J.; et al. Zeaxanthin induces Nrf2-mediated phase II enzymes in protection of cell death. Cell Death Dis. 2014, 5, e1218. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.J.; Lee, M.; Shin, T.; Yoon, N.; Kim, J.H.; Kim, H.R. Eckol enhances heme oxygenase-1 expression activation of Nrf-2/JNK pathway in HepG2 cells. Molecules 2014, 19, 15638–15652. [Google Scholar] [CrossRef] [PubMed]
- Boettler, U.; Volz, N.; Teller, N.; Haupt, L.M.; Bakuradze, T.; Eisenbrand, G.; Bytof, G.; Lantz, I.; Griffiths, L.R.; Marko, D. Induction of antioxidative Nrf2 gene transcription by coffee in humans: Depending on genotype? Mol. Biol. Rep. 2012, 39, 7155–7162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, T.; Hashida, K.; Takarada-Iemata, M.; Matsugo, S.; Hori, O. α-Lipoic acid (LA) enantiomers protect SH-SY5Y cells against glutathione depletion. Neurochem. Int. 2011, 59, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, N.; Yamashita, H.; Gotoh, N.; Yamamoto, Y.; Numano, R.; Niki, E. 2,2′-Azobis (4-methoxy-2,4-dimethylvaleronitrile), a new lipid-soluble azo initiator: Application to oxidations of lipids and low-density lipoprotein in solution and in aqueous dispersions. Free Radic. Biol. Med. 1998, 24, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, A.; Rosta, K.; Pusztai, P.; Tulassay, Z.; Nagy, G. Antioxidant measurements. Physiol. Meas. 2007, 28, R41–R55. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Gao, C.; Luo, M.; Wang, W.; Zhao, C.; Zu, Y.; Efferth, T.; Fu, Y. Dihydroquercetin (DHQ) induced HO-1 and NQO1 expression against oxidative stress through the Nrf2-dependent antioxidant pathway. J. Agric. Food Chem. 2013, 61, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Yang, M.; Lee, S.G.; Davis, C.G.; Kenny, A.; Koo, S.I.; Chun, O.K. Plasma total antioxidant capacity is associated with dietary intake and plasma level of antioxidants in postmenopausal women. J. Nutr. Biochem. 2012, 23, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Z.; Li, J.J.; Chen, C.; Zhang, P.C.; Dong, L.; Chen, J.H.; Chen, Q.; Zhang, X.T.; Wang, Z.L. Protective effects of selenium on oxidative damage and oxidative stress related gene expression in rat liver under chronic poisoning of arsenic. Food Chem. Toxicol. 2013, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hao, H.; Wang, H.; Guo, C.; Kang, A.; Wang, G. Reversing effects of lignans on CCl4-induced hepatic CYP450 down regulation by attenuating oxidative stress. J. Ethnopharmacol. 2014, 155, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, X.; Qiu, X.; Wang, J.; Fang, H.; Wang, Z.; Sun, Y.; Xia, Z. The protective effect of esculentoside A on experimental acute liver injury in mice. PLoS ONE 2014, 9, e113107. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Kim, S.H.; Baek, H.S.; Moon, C.; Kang, S.S.; Kim, S.H.; Kim, Y.B.; Shin, I.S.; Kim, J.C. The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem. Toxicol. 2014, 63, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Q.; Ding, J.; Zhang, L.; Liu, C.M. Protective effects of ursolic acid in an experimental model of liver fibrosis through Nrf2/ARE pathway. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, S.H.; Lee, E.J.; Yoo, C.Y.; Lee, J.K.; Lee, D.Y.; Kim, E.K. Compounds Having an Antioxidant Activity, Method for Preparation Thereof and Composition for Preventing Skin Aging Containing the Same. Korea Patent Registration #10-675552, 23 January 2007. [Google Scholar]
- Kim, S.W.; Lee, S.H.; Heo, J.C.; Lee, E.J.; Kim, D.M. Pharmaceutical and Functional Food Composition for Treating, Preventing or Improving Respiratory Diseases Comprising Amino Acid-Based Derivatives. Korea Patent Registration #10-1076769, 19 November 2011. [Google Scholar]
- Charlton, E. Committee on ethical issues of the international association for the study of pain. Pain 1995, 63, 2778. [Google Scholar]
- Nanjo, F.; Goto, K.; Seto, R.; Suzuki, M.; Sakai, M.; Hara, Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic. Biol. Med. 1996, 21, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.C.; Woo, S.W.; Kweon, M.A.; Park, J.Y.; Lee, H.K.; Son, M.; Rho, J.R.; Lee, S.H. Aqueous extract of a seed from Helianthus annuus alleviates asthmatic symptoms in vivo. Int. J. Mol. Med. 2008, 21, 57–61. [Google Scholar] [PubMed]
- Yoon, E.K.; Kim, H.K.; Cui, S.; Kim, Y.H.; Lee, S.H. Soybean glyceollins mitigate iNOS and COX-2 expression levels via an NF-κB down-regulating signaling pathway in RAW 264.7 cells. Int. J. Mol. Med. 2012, 29, 711–717. [Google Scholar] [PubMed]
- Lebel, C.P.; Bondy, S.C. Sensitive and rapid quantitation of oxygen reactive species formation in rat synaptosomes. Neurochem. Int. 1990, 17, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Ham, H.; Sung, J.; Kim, Y.H.; Choi, Y.; Lee, J.S.; Jeong, S.H.; Lee, J.; Kim, D. Black rice extract protected HepG cells from oxidative stress-induced cell death via ERK1/2 and AKT activation. Nutr. Res. Pract. 2014, 8, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Lautraite, S.; Bigot, L.D.; Bars, R.; Carmichael, N. Optimization of cell-based assays for medium through screening of oxidative stress. Toxicol. In Vitro 2003, 17, 207–220. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, K.; Alam, M.B.; Son, H.-U.; Lee, S.-H. Effect of Novel Compound LX519290, a Derivative of l-allo Threonine, on Antioxidant Potential in Vitro and in Vivo. Int. J. Mol. Sci. 2016, 17, 1451. https://doi.org/10.3390/ijms17091451
Chun K, Alam MB, Son H-U, Lee S-H. Effect of Novel Compound LX519290, a Derivative of l-allo Threonine, on Antioxidant Potential in Vitro and in Vivo. International Journal of Molecular Sciences. 2016; 17(9):1451. https://doi.org/10.3390/ijms17091451
Chicago/Turabian StyleChun, Kun, Md Badrul Alam, Hyeong-U Son, and Sang-Han Lee. 2016. "Effect of Novel Compound LX519290, a Derivative of l-allo Threonine, on Antioxidant Potential in Vitro and in Vivo" International Journal of Molecular Sciences 17, no. 9: 1451. https://doi.org/10.3390/ijms17091451
APA StyleChun, K., Alam, M. B., Son, H. -U., & Lee, S. -H. (2016). Effect of Novel Compound LX519290, a Derivative of l-allo Threonine, on Antioxidant Potential in Vitro and in Vivo. International Journal of Molecular Sciences, 17(9), 1451. https://doi.org/10.3390/ijms17091451