Controlled Synthesis of Polyions of Heavy Main-Group Elements in Ionic Liquids
Abstract
:1. Introduction
2. Polyanions
2.1. Starting Materials with High Vapor Pressure
2.1.1. Polyhalides
2.1.2. Utilization of Metal Carbonyls
2.2. Amine-Assisted Syntheses of Selenidostannates
2.2.1. Promoting Phase Formation
2.2.2. Phase Selectivity
2.2.3. Crystal Growth
3. Polycations
3.1. Homopolycations—Adjustments via Redox Potential and Starting Materials
3.1.1. Bismuth Homopolycations
3.1.2. Tellurium Homopolycations
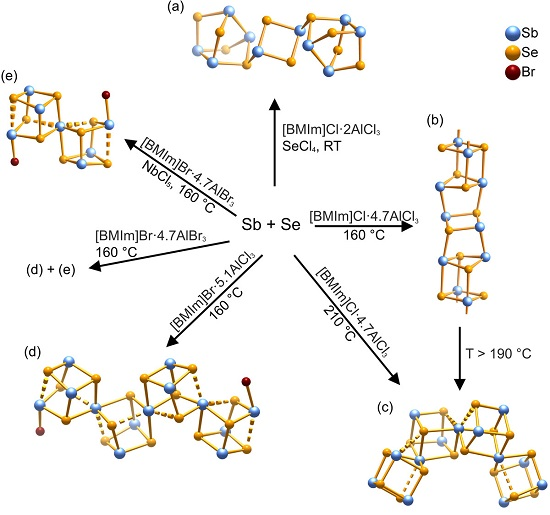
3.2. Antimony-Selenium Heteropolycations—Auxilaries, Temperature, and Influence of Halogens
3.3. Bismuth-Tellurium Heteropolycations—Adjustments via Starting Materials
3.4. Manipulating the Stacking Order of Layered Compounds
3.5. Influence of Concentration and Ion Specification
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Walden, P. Ueber die Molekulargrösse und elektrische Leitfähigkeit einiger geschmolzenen Salze. Izv. Imp. Akad. Nauk 1914, 405–422. (In Germany) [Google Scholar]
- Wilkes, J.S. A short history of ionic liquids—From molten salts to neoteric solvents. Green Chem. 2002, 4, 73–80. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Wasserscheid, P.; Welton, T. Introduction. In Ionic Liquids in Synthesis; Wasserscheid, P., Welton, T., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 1–6. [Google Scholar]
- Ahrens, S.; Peritz, A.; Strassner, T. Tunable Aryl Alkyl Ionic Liquids (TAAILs): The next generation of ionic liquids. Angew. Chem. Int. Ed. 2009, 48, 7908–7910. [Google Scholar] [CrossRef] [PubMed]
- Estager, J.; Holbrey, J.D.; Swadźba-Kwaśny, M. Halometallate ionic liquids—Revisited. Chem. Soc. Rev. 2014, 43, 847–886. [Google Scholar] [CrossRef] [PubMed]
- Mallick, B.; Balke, B.; Felser, C.; Mudring, A.-V. Dysprosium room-temperature ionic liquids with strong luminescence and response to magnetic fields. Angew. Chem. Int. Ed. 2008, 47, 7635–7638. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Griffin, S.T.; Rogers, R.D. Traditional extractants in nontraditional solvents: Groups 1 and 2 extraction by crown ethers in room-temperature ionic liquids. Ind. Eng. Chem. Res. 2000, 39, 3596–3604. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J.H.; Rogers, R.D. Task-specific ionic liquids incorporating novel cations for the coordination and extraction of Hg2+ and Cd2+: Synthesis, characterization, and extraction studies. Environ. Sci. Technol. 2002, 36, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Abai, M.; Atkins, M.P.; Hassan, A.; Holbrey, J.D.; Kuah, Y.; Nockemann, P.; Oliferenko, A.A.; Plechkova, N.V.; Rafeen, S.; Rahman, A.A.; et al. An ionic liquid process for mercury removal from natural gas. Dalton Trans. 2015, 44, 8617–8624. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Liang, Y.; Liu, W. Ionic liquid lubricants: Designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Endres, F.; Bukowski, M.; Hempelmann, R.; Natter, H. Electrodeposition of nanocrystalline metals and alloys from ionic liquids. Angew. Chem. Int. Ed. 2003, 42, 3428–3430. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, N.; Athanassov, Y.; Armand, M.; Bonhôte, P.; Pettersson, H.; Azam, A.; Grätzel, M. The performance and stability of ambient temperature molten salts for solar cell applications. J. Electrochem. Soc. 1996, 143, 3099–3108. [Google Scholar] [CrossRef]
- Pringle, J.M.; Armel, V. The influence of ionic liquid and plastic crystal electrolytes on the photovoltaic characteristics of dye-sensitised solar cells. Int. Rev. Phys. Chem. 2011, 30, 371–407. [Google Scholar] [CrossRef]
- Högberg, D.; Soberats, B.; Uchida, S.; Yoshio, M.; Kloo, L.; Segawa, H.; Kato, T. Nanostructured two-component liquid-crystalline electrolytes for high-temperature dye-sensitized solar cells. Chem. Mater. 2014, 26, 6496–6502. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic liquids for clean technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- Giernoth, R. Task-specific ionic liquids. Angew. Chem. Int. Ed. 2010, 49, 2834–2839. [Google Scholar] [CrossRef] [PubMed]
- Duchet, L.; Legeay, J.C.; Carrié, D.; Paquin, L.; Vanden Eynde, J.J.; Bazureau, J.P. Synthesis of 3,5-disubstituted 1,2,4-oxadiazoles using ionic liquid-phase organic synthesis (IoLiPOS) methodology. Tetrahedron 2010, 66, 986–994. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, C.; Li, C.; Guo, X.; Zhang, S. Carbonyl ruthenium catalysts for the low-temperature water-gas shift reaction with ionic liquids as support structure controllers. Green Chem. 2016. [Google Scholar] [CrossRef]
- Kubisa, P. Ionic liquids as solvents for polymerization processes—Progress and challenges. Prog. Polym. Sci. 2009, 34, 1333–1347. [Google Scholar] [CrossRef]
- Reichert, W.M.; Holbrey, J.D.; Vigour, K.B.; Morgan, T.D.; Broker, G.A.; Rogers, R.D. Approaches to crystallization from ionic liquids: Complex solvents-complex results, or, a strategy for controlled formation of new supramolecular architectures? Chem. Commun. 2006, 4767–4779. [Google Scholar] [CrossRef]
- Tang, S.; Babai, A.; Mudring, A.-V. Europium-based ionic liquids as luminescent soft materials. Angew. Chem. Int. Ed. 2008, 47, 7631–7634. [Google Scholar] [CrossRef] [PubMed]
- Getsis, A.; Tang, S.; Mudring, A.-V. A luminescent ionic liquid crystal: [C12mim]4[EuBr6]Br. Eur. J. Inorg. Chem. 2010, 2010, 2172–2177. [Google Scholar] [CrossRef]
- Ahmed, E.; Breternitz, J.; Groh, M.F.; Ruck, M. Ionic liquids as crystallisation media for inorganic materials. CrystEngComm 2012, 14, 4874–4885. [Google Scholar] [CrossRef]
- Chesman, A.S.R.; Yang, M.; Spiccia, N.D.; Deacon, G.B.; Batten, S.R.; Mudring, A.-V. Lanthanoid-based ionic liquids incorporating the dicyanonitrosomethanide anion. Chem. Eur. J. 2012, 18, 9580–9589. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Terraneo, G.; Monfredini, A.; Saccone, M.; Priimagi, A.; Pilati, T.; Resnati, G.; Metrangolo, P.; Bruce, D.W. Superfluorinated ionic liquid crystals based on supramolecular, halogen-bonded anions. Angew. Chem. Int. Ed. 2016, 55, 6300–6304. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.F.; Müller, U.; Ahmed, E.; Rothenberger, A.; Ruck, M. Substitution of conventional high-temperature syntheses of inorganic compounds by near-room-temperature syntheses in ionic liquids. Z. Naturforsch. 2013, 68b, 1108–1122. [Google Scholar] [CrossRef]
- Ahmed, E.; Ruck, M. Homo and heteroatomic polycations of groups 15 and 16. Recent advances in synthesis and isolation using room temperature ionic liquids. Coord. Chem. Rev. 2011, 255, 2892–2903. [Google Scholar] [CrossRef]
- Freudenmann, D.; Wolf, S.; Wolff, M.; Feldmann, C. Ionic liquids: New perspectives for inorganic synthesis? Angew. Chem. Int. Ed. 2011, 50, 11050–11060. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.F.; Isaeva, A.; Ruck, M. [Ru2Bi14Br4](AlCl4)4 by mobilization and reorganization of complex clusters in ionic liquids. Chem. Eur. J. 2012, 18, 10886–10891. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.F.; Isaeva, A.; Müller, U.; Gebauer, P.; Knies, M.; Ruck, M. Controlled synthesis of pnicogen–chalcogen polycations in ionic liquids. Eur. J. Inorg. Chem. 2016, 2016, 880–889. [Google Scholar] [CrossRef]
- Ahmed, E.; Beck, J.; Daniels, J.; Doert, T.; Eck, S.J.; Heerwig, A.; Isaeva, A.; Lidin, S.; Ruck, M.; Schnelle, W.; et al. A Semiconductor or a One-Dimensional Metal and Superconductor through Tellurium π Stacking. Angew. Chem. Int. Ed. 2012, 51, 8106–8109. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.F.; Knies, M.; Isaeva, A.; Ruck, M. Bi2S3 bipyramids in layered sulfides M2Bi2S3(AlCl4)2 (M = Ag, Cu). Z. Anorg. Allg. Chem. 2015, 641, 279–284. [Google Scholar] [CrossRef]
- Biswas, K.; Zhang, Q.; Chung, I.; Song, J.-H.; Androulakis, J.; Freeman, A.J.; Kanatzidis, M.G. Synthesis in ionic liquids: [Bi2Te2Br](AlCl4), a direct gap semiconductor with a cationic framework. J. Am. Chem. Soc. 2010, 132, 14760–14762. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.F.; Breternitz, J.; Ahmed, E.; Isaeva, A.; Efimova, A.; Schmidt, P.; Ruck, M. Ionothermal synthesis, structure, and bonding of the catena-heteropolycation Sb2Se2]+. Z. Anorg. Allg. Chem. 2015, 641, 388–393. [Google Scholar] [CrossRef]
- Ahmed, E.; Isaeva, A.; Fiedler, A.; Haft, M.; Ruck, M. [Sb10Se10]2+, a heteronuclear polycyclic polycation from a room-temperature ionic liquid. Chem. Eur. J. 2011, 17, 6847–6852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chung, I.; Jang, J.I.; Ketterson, J.B.; Kanatzidis, M.G. Chalcogenide chemistry in ionic liquids: Nonlinear optical wave-mixing properties of the double-cubane compound [Sb7S8Br2](AlCl4)3. J. Am. Chem. Soc. 2009, 131, 9896–9897. [Google Scholar] [CrossRef] [PubMed]
- Freudenmann, D.; Feldmann, C. [Bi3GaS5]2[Ga3Cl10]2[GaCl4]2·S8 containing heterocubane-type [Bi3GaS5]2+, star-shaped [Ga3Cl10]−, monomeric [GaCl4]− and crown-like S8. Dalton Trans. 2010, 40, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Breternitz, J.; Groh, M.F.; Isaeva, A.; Ruck, M. [Sb7Se8Br2]3+ and [Sb13Se16Br2]5+—Double and quadruple spiro cubanes from ionic liquids. Eur. J. Inorg. Chem. 2014, 2014, 3037–3042. [Google Scholar] [CrossRef]
- Feldmann, K.-O.; Wiegand, T.; Ren, J.; Eckert, H.; Breternitz, J.; Groh, M.F.; Müller, U.; Ruck, M.; Maryasin, B.; Ochsenfeld, C.; et al. [P3Se4]+: A binary phosphorus–selenium cation. Chem. Eur. J. 2015, 21, 9697–9712. [Google Scholar] [CrossRef] [PubMed]
- Guloy, A.M.; Ramlau, R.; Tang, Z.; Schnelle, W.; Baitinger, M.; Grin, Y. A guest-free germanium clathrate. Nature 2006, 443, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Taubert, A.; Li, Z. Inorganic materials from ionic liquids. Dalton Trans. 2007, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, C.; Janiak, C. Naked metal nanoparticles from metal carbonyls in ionic liquids: Easy synthesis and stabilization. Coord. Chem. Rev. 2011, 255, 2039–2057. [Google Scholar] [CrossRef]
- Wolff, M.; Meyer, J.; Feldmann, C. [C4MPyr]2[Br20]: Ionic-liquid-based synthesis of a three-dimensional polybromide network. Angew. Chem. Int. Ed. 2011, 50, 4970–4973. [Google Scholar] [CrossRef] [PubMed]
- Du, K.-Z.; Feng, M.-L.; Li, J.-R.; Huang, X.-Y. Ionothermal synthesis and characterization of two cluster chalcohalides: [Cr7S8Cl2(NH3)14.5(H2O)1.5]Cl3·H2O and [Emim]2[Sn2As2S4(S2)2Br2.43Cl1.56]. CrystEngComm 2013, 15, 5594–5597. [Google Scholar] [CrossRef]
- Biswas, K.; Chung, I.; Song, J.-H.; Malliakas, C.D.; Freeman, A.J.; Kanatzidis, M.G. Semiconducting [(Bi4Te4Br2)(Al2Cl6–xBrx)]Cl2 and [Bi2Se2Br](AlCl4): Cationic chalcogenide frameworks from lewis acidic ionic liquids. Inorg. Chem. 2013, 52, 5657–5659. [Google Scholar] [CrossRef] [PubMed]
- Schütte, K.; Meyer, H.; Gemel, C.; Barthel, J.; Fischer, R.A.; Janiak, C. Synthesis of Cu, Zn and Cu/Zn brass alloy nanoparticles from metal amidinate precursors in ionic liquids or propylene carbonate with relevance to methanol synthesis. Nanoscale 2014, 6, 3116–3126. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, S.; Swadźba-Kwaśny, M.; Nockemann, P. Ionothermal, microwave-assisted synthesis of indium(III) selenide. J. Mater. Chem. A 2014, 2, 2616–2622. [Google Scholar] [CrossRef] [Green Version]
- Gebresilassie Eshetu, G.; Armand, M.; Scrosati, B.; Passerini, S. Energy storage materials synthesized from ionic liquids. Angew. Chem. Int. Ed. 2014, 53, 13342–13359. [Google Scholar] [CrossRef] [PubMed]
- Alammar, T.; Chow, Y.-K.; Mudring, A.-V. Energy efficient microwave synthesis of mesoporous Ce0.5M0.5O2 (Ti, Zr, Hf) nanoparticles for low temperature CO oxidation in an ionic liquid—A comparative study. New J. Chem. 2015, 39, 1339–1347. [Google Scholar] [CrossRef]
- Bresien, J.; Ellinger, S.; Harloff, J.; Schulz, A.; Sievert, K.; Stoffers, A.; Täschler, C.; Villinger, A.; Zur Täschler, C. Tetracyanido(difluorido)phosphates M+[PF2(CN)4]−. Angew. Chem. Int. Ed. 2015, 54, 4474–4477. [Google Scholar] [CrossRef] [PubMed]
- Santner, S.; Heine, J.; Dehnen, S. Synthesis of crystalline chalcogenides in ionic liquids. Angew. Chem. Int. Ed. 2016, 55, 876–893. [Google Scholar] [CrossRef] [PubMed]
- Taubert, A. Heavy Elements in Ionic Liquids. In Ionic Liquids; Kirchner, B., Ed.; Topics in Current Chemistry; Springer: Berlin & Heidelberg, Germany, 2009; pp. 127–159. [Google Scholar]
- Janiak, C. Ionic liquids for the synthesis and stabilization of metal nanoparticles. Z. Naturforsch. 2013, 68b, 1059–1089. [Google Scholar] [CrossRef]
- Morris, R.E. Ionothermal Synthesis of Zeolites and Other Porous Materials. In Zeolites and Catalysis; Čejka, J., Corma, A., Zones, S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 87–105. [Google Scholar]
- Mudring, A.-V.; Tang, S. Ionic liquids for lanthanide and actinide chemistry. Eur. J. Inorg. Chem. 2010, 2010, 2569–2581. [Google Scholar] [CrossRef]
- Prechtl, M.H.G.; Campbell, P.S. Metal oxide and bimetallic nanoparticles in ionic liquids: Synthesis and application in multiphase catalysis. Nanotechnol. Rev. 2013, 2, 577–595. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Chen, F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yu, J.; Dai, S. Preparation of inorganic materials using ionic liquids. Adv. Mater. 2010, 22, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Ruck, M. Chemistry of polynuclear transition-metal complexes in ionic liquids. Dalton Trans. 2011, 40, 9347–9357. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.F.; Paasch, S.; Weiz, A.; Ruck, M.; Brunner, E. Unexpected reactivity of red phosphorus in ionic liquids. Eur. J. Inorg. Chem. 2015, 2015, 3991–3994. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, W.; Guan, J.; Xu, Y.; Wang, L.; Ma, H.; Tian, Z.; Han, X.; Lin, L.; Bao, X. New insights into the role of amines in the synthesis of molecular sieves in ionic liquids. Chem. Eur. J. 2009, 15, 5348–5354. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Shi, X.; Zhang, W.; Xu, Y.; Tian, Z.; Lu, X.; Han, X.; Bao, X. Cooperative structure-directing effect in the synthesis of aluminophosphate molecular sieves in ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Ruck, M. Ionothermal synthesis of polyoxometalates. Angew. Chem. Int. Ed. 2012, 51, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Dai, C.; Chen, B. Gas Solubility in Ionic Liquids. Chem. Rev. 2014, 114, 1289–1326. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, J.; Costa Gomes, M.F.; Husson, P.; Majer, V. Solubility of carbon dioxide, ethane, methane, oxygen, nitrogen, hydrogen, argon, and carbon monoxide in 1-butyl-3-methylimidazolium tetrafluoroborate between temperatures 283 K and 343 K and at pressures close to atmospheric. J. Chem. Thermodyn. 2006, 38, 490–502. [Google Scholar] [CrossRef]
- Torralba–Calleja, E.; Skinner, J.; Gutierrez–Tauste, D. CO2 Capture in ionic liquids: A review of solubilities and experimental methods. J. Chem. 2013, 2013, e473584. [Google Scholar] [CrossRef]
- Anthony, J.L.; Anderson, J.L.; Maginn, E.J.; Brennecke, J.F. Anion effects on gas solubility in ionic liquids. J. Phys. Chem. B 2005, 109, 6366–6374. [Google Scholar] [CrossRef] [PubMed]
- Tempel, D.J.; Henderson, P.B.; Brzozowski, J.R.; Pearlstein, R.M.; Cheng, H. High gas storage capacities for ionic liquids through chemical complexation. J. Am. Chem. Soc. 2008, 130, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.; Okrut, A.; Feldmann, C. [(Ph)3PBr][Br7], [(Bz)(Ph)3P]2[Br8], [(n-Bu)3MeN]2[Br20], [C4MPyr]2[Br20], and [(Ph)3PCl]2[Cl2I14]: Extending the horizon of polyhalides via synthesis in ionic liquids. Inorg. Chem. 2011, 50, 11683–11694. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Hog, M.; Scholz, F.; Scherer, H.; Krossing, I.; Riedel, S. [HMIM][Br9]: A Room-temperature ionic liquid based on a polybromide anion. Z. Naturforsch. 2014, 68b, 1103–1107. [Google Scholar] [CrossRef]
- Easton, M.E.; Ward, A.J.; Hudson, T.; Turner, P.; Masters, A.F.; Maschmeyer, T. The formation of high-order polybromides in a room-temperature ionic liquid: From monoanions ([Br5]− to [Br11]−) to the Isolation of [PC16H36]2[Br24] as determined by van der waals bonding radii. Chem. Eur. J. 2015, 21, 2961–2965. [Google Scholar] [CrossRef] [PubMed]
- Easton, M.E.; Ward, A.J.; Chan, B.; Radom, L.; Masters, A.F.; Maschmeyer, T. Factors influencing the formation of polybromide monoanions in solutions of ionic liquid bromide salts. Phys. Chem. Chem. Phys. 2016, 18, 7251–7260. [Google Scholar] [CrossRef] [PubMed]
- Svensson, P.H.; Kloo, L. Synthesis, structure, and bonding in polyiodide and metal iodide–iodine Systems. Chem. Rev. 2003, 103, 1649–1684. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.W.; Burns, G.R.; McKee, V. A new polyhalogen network for bromine—Br102−. Inorg. Chim. Acta 1990, 167, 135–137. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Devillanova, F.A.; Isaia, F.; Lippolis, V.; Mancini, A.; Pala, L.; Slawin, A.M.Z.; Woollins, J.D. First example of an infinite polybromide 2D-network. Chem. Commun. 2003, 2226–2227. [Google Scholar] [CrossRef]
- Haller, H.; Riedel, S. Recent discoveries of polyhalogen anions—From bromine to fluorine. Z. Anorg. Allg. Chem. 2014, 640, 1281–1291. [Google Scholar] [CrossRef]
- CRC Handbook of Chemistry and Physics, 94th ed.; Haynes, W.M. (Ed.) CRC Press: Boca Raton, FL, USA, 2013.
- Sowmiah, S.; Srinivasadesikan, V.; Tseng, M.-C.; Chu, Y.-H. On the chemical stabilities of ionic liquids. Molecules 2009, 14, 3780–3813. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.C.; Dyson, P.J.; Ellis, D.J.; Welton, T. 1-Butyl-3-methylimidazolium cobalt tetracarbonyl [bmim][Co(CO)4]: A catalytically active organometallic ionic liquid. Chem. Commun. 2001, 1862–1863. [Google Scholar] [CrossRef]
- Wolf, S.; Feldmann, C. [(Te2)3{Mn(CO)3}2{Mn(CO)4}3]−—A Novel Tellurium-Manganese Carbonyl with Ufosane-like Structure. Z. Anorg. Allg. Chem. 2012, 638, 1787–1791. [Google Scholar] [CrossRef]
- Wolf, S.; Reiter, K.; Weigend, F.; Klopper, W.; Feldmann, C. [(Pb6I8){Mn(CO)5}6]2−: An Octahedral (M6X8)-like Cluster with Inverted Bonding. Inorg. Chem. 2015, 54, 3989–3994. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Winter, F.; Pöttgen, R.; Middendorf, N.; Klopper, W.; Feldmann, C. [XIm][FeI(CO)3(SnI3)2] (XIm: EMIm, EHIm, PMIm) containing a barbell-shaped FeSn2-carbonyl complex. Dalton Trans. 2012, 41, 10605–10611. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Winter, F.; Pöttgen, R.; Middendorf, N.; Klopper, W.; Feldmann, C. [{Fe(CO)3}4{SnI}6I4]2−: The first bimetallic adamantane-like cluster. Chem. Eur. J. 2012, 18, 13600–13604. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.R.; Andrews, C.D.; Wheatley, P.S.; Webb, P.B.; Wormald, P.; Morris, R.E. Ionic liquids and eutectic mixtures as solvent and template in synthesis of zeolite analogues. Nature 2004, 430, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Parnham, E.R.; Drylie, E.A.; Wheatley, P.S.; Slawin, A.M.Z.; Morris, R.E. Ionothermal materials synthesis using unstable deep-eutectic solvents as template-delivery agents. Angew. Chem. Int. Ed. 2006, 45, 4962–4966. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E. Ionothermal synthesis—Ionic liquids as functional solvents in the preparation of crystalline materials. Chem. Commun. 2009, 2990–2998. [Google Scholar] [CrossRef] [PubMed]
- Wragg, D.S.; Slawin, A.M.Z.; Morris, R.E. The role of added water in the ionothermal synthesis of microporous aluminium phosphates. Solid State Sci. 2009, 11, 411–416. [Google Scholar] [CrossRef]
- Pei, R.; Wei, Y.; Li, K.; Wen, G.; Xu, R.; Xu, Y.; Wang, L.; Ma, H.; Wang, B.; Tian, Z.; et al. Mixed template effect adjusted by amine concentration in ionothermal synthesis of molecular sieves. Dalton Trans. 2010, 39, 1441–1443. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Y.; Wei, Y.; Duan, J.; Chen, A.; Wang, B.; Ma, H.; Tian, Z.; Lin, L. Structure-Directing Role of Amines in the Ionothermal Synthesis. J. Am. Chem. Soc. 2006, 128, 7432–7433. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Xie, Z.-L.; He, X.-W.; Li, L.-H.; Huang, X.-Y. Crystalline Open-Framework Selenidostannates Synthesized in Ionic Liquids. Angew. Chem. Int. Ed. 2011, 50, 11395–11399. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Massa, W.; Dehnen, S. “Zeoball” [Sn36Ge24Se132]24−: A molecular anion with zeolite-related composition and spherical shape. J. Am. Chem. Soc. 2012, 134, 4497–4500. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Massa, W.; Dehnen, S. Controlling the assembly of chalcogenide anions in ionic liquids: From binary Ge/Se through ternary Ge/Sn/Se to binary Sn/Se frameworks. Chem. Eur. J. 2012, 18, 13427–13434. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xie, D.; Massa, W.; Mayrhofer, L.; Lippert, S.; Ewers, B.; Chernikov, A.; Koch, M.; Dehnen, S. Changes in the structural dimensionality of selenidostannates in ionic liquids: Formation, structures, stability, and photoconductivity. Chem. Eur. J. 2013, 19, 8806–8813. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Xiong, W.-W.; Xie, Z.-L.; Du, C.-F.; Zou, G.-D.; Huang, X.-Y. From selenidostannates to silver-selenidostannate: Structural variation of chalcogenidometallates synthesized in ionic liquids. Chem. Commun. 2012, 49, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Santner, S.; Dehnen, S. [M4Sn4Se17]10− Cluster Anions (M = Mn, Zn, Cd) in a Cs+ Environment and as ternary precursors for ionothermal treatment. Inorg. Chem. 2015, 54, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Du, C.-F.; Li, J.-R.; Feng, M.-L.; Zou, G.-D.; Shen, N.-N.; Huang, X.-Y. Varied forms of lamellar [Sn3Se7]n2n− anion: The competitive and synergistic structure-directing effects of metal–amine complex and imidazolium cations. Dalton Trans. 2015, 44, 7364–7372. [Google Scholar] [CrossRef] [PubMed]
- Du, C.-F.; Shen, N.-N.; Li, J.-R.; Hao, M.-T.; Wang, Z.; Cheng, C.-C.; Huang, X.-Y. Two novel selenidostannates from mixed structure-directing systems: The large ten-membered ring of [Sn3Se4] semicubes and the 3D [Sn4Se9]n2n− with multi-channels. Dalton Trans. 2016, 45, 9523–9528. [Google Scholar] [CrossRef] [PubMed]
- Du, C.-F.; Shen, N.-N.; Li, J.-R.; Hao, M.-T.; Wang, Z.; Huang, X.-Y. Synthesizing 2D and 3D selenidostannates in ionic liquids: The synergistic structure-directing effects of ionic liquids and metal–amine complexes. Chem. Asian J. 2016, 11, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Köhler, D.; Ruck, M. Room-temperature synthesis of bismuth clusters in ionic liquids and crystal growth of Bi5(AlCl4)3. Z. Anorg. Allg. Chem. 2009, 635, 297–300. [Google Scholar] [CrossRef]
- Boxall, L.G.; Jones, H.L.; Osteryoung, R.A. Solvent equilibria of AlCl3–NaCl melts. J. Electrochem. Soc. 1973, 120, 223–231. [Google Scholar] [CrossRef]
- Ahmed, E.; Ahrens, E.; Heise, M.; Ruck, M. A facile route for the synthesis of polycationic tellurium cluster compounds: Synthesis in ionic liquid media and characterization by single-crystal X-ray crystallography and magnetic susceptibility. Z. Anorg. Allg. Chem. 2010, 636, 2602–2606. [Google Scholar] [CrossRef]
- Schulz, C.; Daniels, J.; Bredow, T.; Beck, J. The electrochemical synthesis of polycationic clusters. Angew. Chem. Int. Ed. 2016, 55, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Freudenmann, D.; Feldmann, C. [Te8]2[Ta4O4Cl16]: A Two-dimensional tellurium polycation obtained via ionic liquid-based synthesis. Z. Anorg. Allg. Chem. 2011, 637, 1481–1485. [Google Scholar] [CrossRef]
- Åkerstedt, J.; Gorlov, M.; Kloo, L. Room-temperature synthesis of the Bi5[GaCl4]3 salt from three different classes of ionic liquids. J. Cluster Sci. 2012, 24, 157–164. [Google Scholar] [CrossRef]
- Beck, J.; Dolg, M.; Schlüter, S. Bi4Te44+—A cube-shaped, polycationic main group element cluster. Angew. Chem. Int. Ed. 2001, 40, 2287–2290. [Google Scholar] [CrossRef]
- Heerwig, A.; Ruck, M. The Low-valent bismuth sulfide bromide Cu3Bi2S3Br2. Z. Anorg. Allg. Chem. 2011, 637, 1814–1817. [Google Scholar] [CrossRef]
- Groh, M.F.; Ruck, M. Mobilization and modification of intermetalloid clusters in ionic liquids. Z. Anorg. Allg. Chem. 2014, 640, 2391. [Google Scholar]
- Dubenskyy, V.; Ruck, M. Das Subchlorid Bi16PdCl22: Pd@Bi104+-polykationen in einem raumnetzwerk aus chlorobismutat(III)-anionen. Z. Anorg. Allg. Chem. 2004, 630, 2458–2462. [Google Scholar] [CrossRef]
- Groh, M.F.; Wolff, A.; Wahl, B.; Rasche, B.; Gebauer, P.; Ruck, M. Pentagonal Bismuth antiprisms with endohedral palladium or platinum atoms by low-temperature syntheses. Chem. Eur. J. 2016. submitted. [Google Scholar]
- Blander, M.; Bierwagen, E.; Calkins, K.G.; Curtiss, L.A.; Price, D.L.; Saboungi, M.-L. Structure of acidic haloaluminate melts: Neutron diffraction and quantum chemical calculations. J. Chem. Phys. 1992, 97, 2733–2741. [Google Scholar] [CrossRef]






| Abbreviation | Full Name |
|---|---|
| [EHIm] | 1-ethylimidazolium |
| [EMIm] | 1-ethyl-3-methylimidazolium |
| [PMIm] | 1-propyl-3-methylimidazolium |
| [BMIm] | 1-butyl-3-methylimidazolium |
| [HMIm] | 1-hexyl-3-methylimidazolium |
| [DMIm] | 1-dodecyl-3-methylimidazolium |
| [BMMIm] | 1-butyl-2,3-dimethylimidazolium |
| [PMMIm] | 1-propyl-2,3-dimethylimidazolium |
| [C4MPyr] | butyl-methylpyrrolidinium |
| [C10MPyr] | decyl-methylpyrrolidinium |
| [N(n-Bu)3Me] | tributylmethylammonium |
| [P4444] | tetrabutylphosphonium |
| [P66614] | trihexyl-tetradecylphosphonium |
| [P(Bz)(Ph)3] | benzyl(triphenyl)phosphonium |
| [CTf3] | tristriflylmethanide, tris(trifluoromethanesulfonyl)methanimide |
| [NTf2] | triflimide, bis(trifluoromethanesulfonyl)imide |
| [OTf] | triflate, trifluoromethanesulfonate |
| Carbonyl | Iodide | IL | Cluster Anion | Ref. |
|---|---|---|---|---|
| Mn2(CO)10 | TeI4 | [BMIm][OTf] | [(Te2)3{Mn(CO)3}2{Mn(CO)4}3]− | [80] |
| PbI2 | [BMIm][NTf2] | [(Pb6I8){Mn(CO)5}6]2− | [81] | |
| Fe(CO)5 | SnI4 | [EMIm][NTf2] | [FeI(CO)3(SnI3)2]− | [82] |
| [EHIm][NTf2] | [FeI(CO)3(SnI3)2]− | [82] | ||
| [PMIm][NTf2] | [FeI(CO)3(SnI3)2]− | [82] | ||
| [BMIm][NTf2] | [{Fe(CO)3}4Sn6I10]2− | [83] | ||
| [BMIm][OTf] | [{Fe(CO)3}4Sn6I10]2− | [83] |
| V/μL | 2,6-Dimethylmorpholine = DMMP | ethylenediamine = en |
|---|---|---|
| 0 | – | – |
| 10 | – | 0D-[BMMIm]24[Sn36Ge24Se132] |
| 20 | – | 0D-[BMMIm]24[Sn36Ge24Se132] |
| 30 | 0D-[BMMIm]24[Sn36Ge24Se132] | 2D-[BMMIm]2[Ge0.83Sn3.17Se9.06] |
| 50 | 0D-[BMMIm]24[Sn36Ge24Se132] | 2D-[BMMIm]2[Ge0.83Sn3.17Se9.06] |
| 100 | 0D-[BMMIm]24[Sn36Ge24Se132] | 3D-[BMMIm]8[Sn18Se40] |
| 2D-[BMMIm]2[Ge0.83Sn3.17Se9.06] | ||
| >200 | microcrystalline SnSe2 | – |
| n(IL):n(hydrazine hydrate) | Product |
|---|---|
| (without amine) | 3D-[PMMIm]4[Sn9Se20.93]-nanoparticles |
| 4.9:1.0 | 3D-[PMMIm]4[Sn9Se20.93] |
| 2D-[PMMIm]8[Sn17Se38] | |
| 4.9:1.6 | 2D-[PMMIm]8[Sn17Se38] |
| 2.0:15.0 | 2D-[PMMIm]2[Sn3Se7] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groh, M.F.; Wolff, A.; Grasser, M.A.; Ruck, M. Controlled Synthesis of Polyions of Heavy Main-Group Elements in Ionic Liquids. Int. J. Mol. Sci. 2016, 17, 1452. https://doi.org/10.3390/ijms17091452
Groh MF, Wolff A, Grasser MA, Ruck M. Controlled Synthesis of Polyions of Heavy Main-Group Elements in Ionic Liquids. International Journal of Molecular Sciences. 2016; 17(9):1452. https://doi.org/10.3390/ijms17091452
Chicago/Turabian StyleGroh, Matthias F., Alexander Wolff, Matthias A. Grasser, and Michael Ruck. 2016. "Controlled Synthesis of Polyions of Heavy Main-Group Elements in Ionic Liquids" International Journal of Molecular Sciences 17, no. 9: 1452. https://doi.org/10.3390/ijms17091452
APA StyleGroh, M. F., Wolff, A., Grasser, M. A., & Ruck, M. (2016). Controlled Synthesis of Polyions of Heavy Main-Group Elements in Ionic Liquids. International Journal of Molecular Sciences, 17(9), 1452. https://doi.org/10.3390/ijms17091452