Advances in Integrating Traditional and Omic Biomarkers When Analyzing the Effects of the Mediterranean Diet Intervention in Cardiovascular Prevention
Abstract
:1. Introduction
2. Advances in Nutrition: Traditional Mediterranean Diet and Cardiovascular Risk
3. Effects of the MedDiet on Traditional Biomarkers (Classical and Non-Classical Risk Factors)
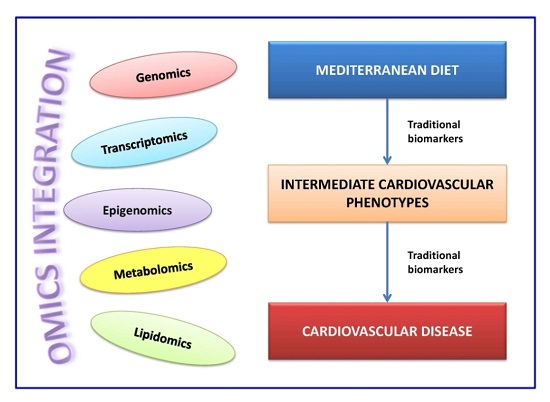
4. Omics Integration and New Omic-Based Biomarkers
5. Omic Biomarkers in the PREDIMED Study and in Other Studies: Gene–MedDiet Interactions
5.1. Genomic Biomarkers
5.2. Epigenomic Biomarkers
5.3. Transcriptomic Biomarkers
5.4. Metabolomic Biomarkers
5.5. Lipidomic Biomarkers
5.6. Multi-Omics Integration in the Response to MedDiet and Cardiovascular Risk
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.Á.; Toledo, E.; Arós, F.; Fiol, M.; Corella, D.; Salas-Salvadó, J.; Ros, E.; Covas, M.I.; Fernández-Crehuet, J.; Lapetra, J.; et al. Extravirgin olive oil consumption reduces risk of atrial fibrillation: The PREDIMED (Prevención con Dieta Mediterránea) trial. Circulation 2014, 130, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.Á.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Estruch, R.; Corella, D.; Salas-Salvadó, J.; Martínez-González, M.A. Association of Mediterranean diet with peripheral artery disease: The PREDIMED randomized trial. JAMA 2014, 311, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, S.S. Toward a road map for global-omics: A primer on -omic technologies. Am. J. Epidemiol. 2014, 180, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Ordovás, J.M. Biomarkers: Background, classification and guidelines for applications in nutritional epidemiology. Nutr. Hosp. 2015, 31, 177–188. [Google Scholar] [PubMed]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Chiva-Blanch, G.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; Lapetra, J.; et al. Lamuela-Raven effect of a high-fat Mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2016, 4, 666–676. [Google Scholar] [CrossRef]
- Solá, R.; Fitó, M.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; de La Torre, R.; Muñoz, M.A.; López-Sabater, M.C.; Martínez-González, M.A.; Arós, F.; et al. Effect of a traditional Mediterranean diet on apolipoproteins B, A-I, and their ratio: A randomized, controlled trial. Atherosclerosis 2011, 218, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, N.R.; Sala-Vila, A.; Cofán, M.; Pérez-Heras, A.M.; Fitó, M.; Ruiz-Gutiérrez, V.; Martínez-González, M.A.; Corella, D.; Arós, F.; Estruch, R.; et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis 2013, 230, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Mekki, K.; Bouzidi-bekada, N.; Kaddous, A.; Bouchenak, M. Mediterranean diet improves dyslipidemia and biomarkers in chronic renal failure patients. Food Funct. 2010, 1, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Kakafika, A.I.; Papageorgiou, A.A.; Tziomalos, K.; Peletidou, A.; Vosikis, C.; Karagiannis, A.; Mikhailidis, D.P. Effect of a plant stanol ester-containing spread, placebo spread, or Mediterranean diet on estimated cardiovascular risk and lipid, inflammatory and haemostatic factors. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kastorini, C.M.; Haralampos, J.M.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components a meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1213. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Huelgas, R.; Jansen-Chaparro, S.; Baca-Osorio, A.J.; Mancera-Romero, J.; Tinahones, F.J.; Bernal-López, M.R. Effects of a long-term lifestyle intervention program with Mediterranean diet and exercise for the management of patients with metabolic syndrome in a primary care setting. Eur. J. Intern. Med. 2015, 26, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Bihuniak, J.D.; Shook, J.; Kenny, A.; Kerstetter, J.; Huedo-Medina, T.B. The effect of the traditional Mediterranean-style diet on metabolic risk factors: A meta-analysis. Nutrients 2016, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Perez-Martinez, P.; Garcia-Rios, A.; Alcala-Diaz, J.F.; Perez-Caballero, A.I.; Gomez-Delgado, F.; Fuentes, F.; Quintana-Navarro, G.; Lopez-Segura, F.; Ortiz-Morales, A.M.; et al. CORonary Diet Intervention with Olive oil and cardiovascular PREVention study (the CORDIOPREV study): Rationale, methods, and baseline characteristics: A clinical trial comparing the efficacy of a Mediterranean diet rich in olive oil versus a low-fat diet. Am. Heart J. 2016, 177, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Moller, C.S.; Zethelius, B.; Sundstrom, J.; Lind, L. Impact of follow-up time and re-measurement of the electrocardiogram and conventional cardiovascular risk factors on their predictive value for myocardial infarction. J. Intern. Med. 2006, 260, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Valle, V.; Arós, F.; Sanz, G.; Sala, J.; Fiol, M.; Bruguera, J.; Elosua, R.; Molina, L.; Martí, H.; et al. Oxidized LDL, lipoprotein (a) and other emergent risk factors in acute myocardial infarction (FORTIAM study). Rev. Esp. Cardiol. 2009, 62, 373–382. [Google Scholar] [CrossRef]
- Khot, U.N.; Khot, M.B.; Bajzer, C.T.; Sapp, S.K.; Ohman, E.M.; Brener, S.J.; Ellis, S.G.; Lincoff, A.M.; Topol, E.J. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 2003, 290, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Fiol, M.; Cabadés, A.; Sala, J.; Marrugat, J.; Elosua, R.; Vega, G.; José Tormo Díaz, M.; Segura, A.; Aldasoro, E.; Moreno-Iribas, C.; et al. Variability in the in-hospital management of acute myocardial infarction in Spain: IBERICA Study. Rev. Esp. Cardiol. 2001, 54, 443–452. [Google Scholar] [CrossRef]
- Björnson, E.; Borén, J.; Mardinoglu, A. Personalized cardiovascular disease prediction and treatment—A review of existing strategies and novel systems medicine tools. Front. Physiol. 2016, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef]
- Witzum, J. The oxidation hypothesis of atherosclerosis. Lancet 1994, 349, 793–795. [Google Scholar] [CrossRef]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otsuka, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T.; et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Vila, J.; Elosua, R.; Molina, L.; Bruguera, J.; Sala, J.; Masià, R.; Covas, M.I.; Marrugat, J.; Fitó, M. Relationship of lipid oxidation with subclinical atherosclerosis and 10-year coronary events in general population. Atherosclerosis 2014, 232, 134–140. [Google Scholar]
- Fitó, M.; Guxens, M.; Corella, D.; Sáez, G.; Estruch, R.; de la Torre, R.; Francés, F.; Cabezas, C.; López-Sabater Mdel, C.; Marrugat, J.; et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: A randomized controlled trial. Arch. Intern. Med. 2007, 167, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M.; Estruch, R.; Salas-Salvadó, J.; Martínez-Gonzalez, M.A.; Arós, F.; Vila, J.; Corella, D.; Díaz, O.; Sáez, G.; de la Torre, R.; et al. Effect of the Mediterranean diet on heart failure biomarkers: A randomized sample from the PREDIMED trial. Eur. J. Heart Fail. 2014, 16, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Mitjavila, M.T.; Fandos, M.; Salas-Salvadó, J.; Covas, M.I.; Borrego, S.; Estruch, R.; Lamuela-Raventós, R.; Corella, D.; Martínez-Gonzalez, M.Á.; Sánchez, J.M.; et al. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals: A randomized, controlled, trial. Clin. Nutr. 2013, 32, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Serafini, M.; Estruch, R.; Lamuela-Raventós, R.M.; Martínez-González, M.A.; Salas-Salvadó, J.; Fiol, M.; Lapetra, J.; Arós, F.; Covas, M.I.; et al. Mediterranean diet and non enzymatic antioxidant capacity in the PREDIMED study: Evidence for a mechanism of antioxidant tuning. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Razquin, C.; Martinez, J.A.; Martinez-Gonzalez, M.A.; Mitjavila, M.T.; Estruch, R.; Marti, A. A 3 years follow-up of a Mediterranean diet rich in virgin olive oil is associated with high plasma antioxidant capacity and reduced body weight gain. Eur. J. Clin. Nutr. 2009, 63, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Martinez, M.; Palacios, M.; Martinez-Losa, E.; Lezaun, R.; Maravi, C.; Prado, M.; Martínez, J.A.; Martinez-Gonzalez, M.A. A Mediterranean dietary style influences TNF-α and VCAM-1 coronary blood levels in unstable angina patients. Eur. J. Nutr. 2005, 44, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.; Zulet, M.Á.; Abete, I.; Martínez, J.A. Discriminated benefits of a Mediterranean dietary pattern within a hypocaloric diet program on plasma RBP4 concentrations and other inflammatory markers in obese subjects. Endocrine 2009, 36, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Corella, D.; Castañer, O.; Lamuela-Raventos, R.M.; Salas-Salvadó, J.; Martínez-González, M.A.; Ros, E.; Estruch, R. Long-term immunomodulatory effects of a mediterranean diet in adults at high risk of cardiovascular disease in the PREvención con Dieta MEDiterránea randomized controlled trial. J. Nutr. 2016, 146, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Capurso, C.; Massaro, M.; Scoditti, E.; Vendemiale, G.; Capurso, A. Vascular effects of the Mediterranean diet part I: Anti-hypertensive and anti-thrombotic effects. Vascul. Pharmacol. 2014, 63, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Garcia-Rios, A.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F. Olive oil and haemostasis: Platelet function, thrombogenesis and fibrinolysis. Curr. Pharm. Des. 2011, 17, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Fernández de la Puebla, R.A.; Pérez-Martínez, P.; Carmona, J.; López-Miranda Carmen Marín, J.; Paniagua, J.A.; Fuentes, F.; Pérez-Jiménez, F. Factor VII polymorphisms influence the plasma response to diets with different fat content, in a healthy caucasian population. Mol. Nutr. Food Res. 2007, 51, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, H.E.; Koeller, E.; Greer, N.; MacDonald, R.; Kane, R.; Wilt, T.J. Effects on health outcomes of a Mediterranean diet with no restriction on fat intake: A systematic review and meta-analysis. Ann. Intern. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, S.; Srivastava, D. Genetics of human cardiovascular disease. Cell 2012, 148, 1242–1257. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R. Genetics of coronary artery disease. Circ. Res. 2014, 114, 1890–1903. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Capurso, C.; Capurso, A.; Massaro, M. Vascular effects of the Mediterranean diet-part II: Role of ω-3 fatty acids and olive oil polyphenols. Vascul. Pharmacol. 2014, 63, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Jaureguiberry, M.S.; Tricerri, M.A.; Sanchez, S.A.; Finarelli, G.S.; Montanaro, M.A.; Prieto, E.D.; Rimoldi, O.J. Role of plasma membrane lipid composition on cellular homeostasis: Learning from cell line models expressing fatty acid desaturases. Acta Biochim. Biophys. Sin. 2014, 46, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, B.A.; Baker, P.R.; Schopfer, F.J.; Woodcock, S.R.; Napolitano, A.; d'Ischia, M. Nitro-fatty acid formation and signaling. J. Biol. Chem. 2008, 283, 15515–15519. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, K.M.; Fava, F.; Viola, R. The way to a man’s heart is through his gut microbiota’—Dietary pro- and prebiotics for the management of cardiovascular risk. Proc. Nutr. Soc. 2014, 73, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Legarrea, P.; Fuller, N.R.; Zulet, M.A.; Martinez, J.A.; Caterson, I.D. The influence of Mediterranean, carbohydrate and high protein diets on gut microbiota composition in the treatment of obesity and associated inflammatory state. Asia Pac. J. Clin. Nutr. 2014, 23, 360–368. [Google Scholar] [PubMed]
- Zulet, M.A.; Bondia-Pons, I.; Abete, I.; de la Iglesia, R.; López-Legarrea, P.; Forga, L.; Navas-Carretero, S.; Martínez, J.A. The reduction of the metabolyc syndrome in Navarra-Spain (RESMENA-S) study: A multidisciplinary strategy based on chrononutrition and nutritional education, together with dietetic and psychological control. Nutr. Hosp. 2011, 26, 16–26. [Google Scholar] [PubMed]
- Müller, M.; Kersten, S. Nutrigenomics: Goals and strategies. Nat. Rev. Genet. 2003, 4, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Ordovás, J.M. How does the Mediterranean diet promote cardiovascular health? Current progress toward molecular mechanisms: Gene-diet interactions at the genomic, transcriptomic, and epigenomic levels provide novel insights into new mechanisms. Bioessays 2014, 36, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Van der Sijde, M.R.; Ng, A.; Fu, J. Systems genetics: From GWAS to disease pathways. Biochim. Biophys. Acta 2014, 1842, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.; Bhalala, O.G.; de Bakker, P.I.; Ripatti, S.; Inouye, M. Towards a molecular systems model of coronary artery disease. Curr. Cardiol. Rep. 2014, 16, 488. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Arregui, M.; Coltell, O.; Portolés, O.; Guillem-Sáiz, P.; Carrasco, P.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Ordovás, J.M. Association of the LCT-13910 C>T polymorphism with obesity and its modulation by dairy products in a Mediterranean population. Obesity 2011, 19, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Park, L.K.; Friso, S.; Choi, S.W. Nutritional influences on epigenetics and age-related disease. Proc. Nutr. Soc. 2012, 71, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Aslibekyan, S.; Claas, S.A.; Arnett, D.K. Clinical applications of epigenetics in cardiovascular disease: The long road ahead. Transl. Res. 2015, 165, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Siemelink, M.A.; Zeller, T. Biomarkers of coronary artery disease: The promise of the transcriptome. Curr. Cardiol. Rep. 2014, 16, 513. [Google Scholar] [CrossRef] [PubMed]
- Castañer, O.; Corella, D.; Covas, M.I.; Sorlí, J.V.; Subirana, I.; Flores-Mateo, G.; Nonell, L.; Bulló, M.; de la Torre, R.; Portolés, O.; et al. In vivo transcriptomic profile after a Mediterranean diet in high-cardiovascular risk patients: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 98, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Arab, S.; Gramolini, A.O.; Ping, P.; Kislinger, T.; Stanley, B.; van Eyk, J.; Ouzounian, M.; MacLennan, D.H.; Emili, A.; Liu, P.P. Cardiovascular proteomics: Tools to develop novel biomarkers and potential applications. J. Am. Coll. Cardiol. 2006, 48, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Hinterwirth, H.; Stegemann, C.; Mayr, M. Lipidomics: Quest for molecular lipid biomarkers in cardiovascular disease. Circ. Cardiovasc. Genet. 2014, 7, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Ganna, A.; Salihovic, S.; Sundström, J.; Broeckling, C.D.; Hedman, A.K.; Magnusson, P.K.; Pedersen, N.L.; Larsson, A.; Siegbahn, A.; Zilmer, M.; et al. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS Genet. 2014, 10, e1004801. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Fresno, R.; Llorach, R.; Urpi-Sarda, M.; Lupianez-Barbero, A.; Estruch, R.; Corella, D.; Fitó, M.; Arós, F.; Ruiz-Canela, M.; Salas-Salvadó, J.; et al. Metabolomic pattern analysis after Mediterranean diet intervention in a nondiabetic population: A 1- and 3-year follow-up in the PREDIMED study. J. Proteome Res. 2015, 14, 531–540. [Google Scholar]
- Corella, D.; Carrasco, P.; Fitó, M.; Martínez-González, M.A.; Salas-Salvadó, J.; Arós, F.; Lapetra, J.; Guillén, M.; Ortega-Azorín, C.; Warnberg, J.; et al. Gene-environment interactions of CETP gene variation in a high cardiovascular risk Mediterranean population. J. Lipid Res. 2010, 51, 2798–2807. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Guillén, M.; Vicente Sorli, J.; Portolés, O.; Guillem-Saiz, P.; Ignacio Gonzalez, J.; Qi, L.; Corella, D. Relevant associations of the glucokinase regulatory protein/glucokinase gene variation with TAG concentrations in a high-cardiovascular risk population: Modulation by the Mediterranean diet. Br. J. Nutr. 2013, 109, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Carrasco, P.; Sorlí, J.V.; Estruch, R.; Rico-Sanz, J.; Martínez-González, M.Á.; Salas-Salvadó, J.; Covas, M.I.; Coltell, O.; Arós, F.; et al. Mediterranean diet reduces the adverse effect of the TCF7L2-rs7903146 polymorphism on cardiovascular risk factors and stroke incidence: A randomized controlled trial in a high-cardiovascular-risk population. Diabetes Care 2013, 36, 3803–3811. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Azorín, C.; Sorlí, J.V.; Estruch, R.; Asensio, E.M.; Coltell, O.; González, J.I.; Martínez-González, M.Á.; Ros, E.; Salas-Salvadó, J.; Fitó, M.; et al. Amino acid change in the carbohydrate response element binding protein is associated with lower triglycerides and myocardial infarction incidence depending on level of adherence to the Mediterranean diet in the PREDIMED trial. Circ. Cardiovasc. Genet. 2014, 7, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Azorín, C.; Sorlí, J.V.; Asensio, E.M.; Coltell, O.; Martínez-González, M.Á.; Salas-Salvadó, J.; Covas, M.I.; Arós, F.; Lapetra, J.; Serra-Majem, L.; et al. Associations of the FTO rs9939609 and the MC4R rs17782313 polymorphisms with type 2 diabetes are modulated by diet, being higher when adherence to the Mediterranean diet pattern is low. Cardiovasc. Diabetol. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; González, J.I.; Bulló, M.; Carrasco, P.; Portolés, O.; Díez-Espino, J.; Covas, M.I.; Ruíz-Gutierrez, V.; Gómez-Gracia, E.; Arós, F.; et al. Polymorphisms cyclooxygenase-2-765G>C and interleukin-6-174G>C are associated with serum inflammation markers in a high cardiovascular risk population and do not modify the response to a Mediterranean diet supplemented with virgin olive oil or nuts. J. Nutr. 2009, 139, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Guillén, M.; Portolés, O.; Sorlí, J.V.; González, J.I.; Asensio, E.M.; Corella, D. Association between the rs6950982 polymorphism near the SERPINE1 gene and blood pressure and lipid parameters in a high-cardiovascular-risk population: Interaction with Mediterranean diet. Genes Nutr. 2013, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Ortega-Azorín, C.; Sorlí, J.V.; Covas, M.I.; Carrasco, P.; Salas-Salvadó, J.; Martínez-González, M.Á.; Arós, F.; Lapetra, J.; Serra-Majem, L.; et al. Statistical and biological gene-lifestyle interactions of MC4R and FTO with diet and physical activity on obesity: New effects on alcohol consumption. PLoS ONE 2012, 7, e52344. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Quattrocchi, A.; Adornetto, V.; Marchese, A.E.; Agodi, A. Tumor necrosis factor-α-308 G>A polymorphism, adherence to Mediterranean diet, and risk of overweight/obesity in young women. Biomed. Res. Int. 2014, 2014, 742620. [Google Scholar] [CrossRef] [PubMed]
- Roswall, N.; Ängquist, L.; Ahluwalia, T.S.; Romaguera, D.; Larsen, S.C.; Østergaard, J.N.; Halkjaer, J.; Vimaleswaran, K.S.; Wareham, N.J.; Bendinelli, B.; et al. Association between Mediterranean and Nordic diet scores and changes in weight and waist circumference: Influence of FTO and TCF7L2 loci. Am. J. Clin. Nutr. 2014, 100, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Pitsavos, C.; Panagiotakos, D.; Trichopoulou, A.; Chrysohoou, C.; Dedoussis, G.; Chloptsios, Y.; Choumerianou, D.; Stefanadis, C. Interaction between Mediterranean diet and methylenetetrahydrofolate reductase C677T mutation on oxidized low density lipoprotein concentrations: The ATTICA study. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Delgado, F.; Alcala-Diaz, J.F.; Garcia-Rios, A.; Delgado-Lista, J.; Ortiz-Morales, A.; Rangel-Zuñiga, O.; Tinahones, F.J.; Gonzalez-Guardia, L.; Malagon, M.M.; Bellido-Muñoz, E.; et al. Polymorphism at the TNF-α gene interacts with Mediterranean diet to influence triglyceride metabolism and inflammation status in metabolic syndrome patients: From the CORDIOPREV clinical trial. Mol. Nutr. Food Res. 2014, 58, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N.; di Renzo, L.; Noce, A.; Iacopino, L.; Ferraro, P.M.; Rizzo, M.; Sarlo, F.; Domino, E.; de Lorenzo, A. Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. J. Nephrol. 2014, 27, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Sorlí, J.V.; Estruch, R.; Coltell, O.; Ortega-Azorín, C.; Portolés, O.; Martínez-González, M.A.; Bulló, M.; Fitó, M.; Arós, F.; et al. MicroRNA-410 regulated lipoprotein lipase variant rs13702 is associated with stroke incidence and modulated by diet in the randomized controlled PREDIMED trial. Am. J. Clin. Nutr. 2014, 100, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Asensio, E.M.; Coltell, O.; Sorlí, J.V.; Estruch, R.; Martínez-González, M.Á.; Salas-Salvadó, J.; Castañer, O.; Arós, F.; Lapetra, J.; et al. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: Dietary modulation in the PREDIMED randomized trial. Cardiovasc. Diabetol. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.G.; Marquezine, G.F.; Lemos, P.A.; Martinez, E.; Lopes, N.; Hueb, W.A.; Krieger, J.E.; Pereira, A.C. TCF7L2 polymorphism rs7903146 is associated with coronary artery disease severity and mortality. PLoS ONE 2009, 4, e7697. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Lee, D.H.; Jeon, H.J.; Kim, D.S.; Lee, Y.H.; Oh, T. Transcription factor 7-like 2 (TCF7L2) gene polymorphism rs7903146 is associated with stroke in type 2 diabetes patients with long disease duration. Diabetes Res. Clin. Pract. 2014, 103, e3–e6. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, S.; Melander, O.; Guiducci, C.; Surti, A.; Burtt, N.P.; Rieder, M.J.; Cooper, G.M.; Roos, C.; Voight, B.F.; Havulinna, A.S.; et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 2008, 40, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Kooner, J.S.; Chambers, J.C.; Aguilar-Salinas, C.A.; Hinds, D.A.; Hyde, C.L.; Warnes, G.R.; Gómez Pérez, F.J.; Frazer, K.A.; Elliott, P.; Scott, J.; et al. Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nat. Genet. 2008, 40, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Barrès, R.; Zierath, J.R. The role of diet and exercise in the transgenerational epigenetic landscape of T2DM. Nat. Rev. Endocrinol. 2016, 12, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Rönn, T.; Ling, C. DNA methylation as a diagnostic and therapeutic target in the battle against Type 2 diabetes. Epigenomics 2015, 7, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Abete, I.; Gomez-Uriz, A.M.; Mansego, M.L.; de Arce, A.; Goyenechea, E.; Blazquez, V.; Martinez-Zabaleta, M.T.; Gonzalez-Muniesa, P.; Lopez de Munain, A.; Martinez, J.A.; et al. Epigenetic changes in the methylation patterns of KCNQ1 and WT1 after a weight loss intervention program in obese stroke patients. Curr. Neurovasc. Res. 2015, 12, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Canto, C.; Marchese, A.E.; Vinciguerra, M. Low fruit consumption and folate deficiency are associated with LINE-1 hypomethylation in women of a cancer-free population. Genes Nutr. 2015, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Martín-Núñez, G.M.; Cabrera-Mulero, R.; Rubio-Martín, E.; Rojo-Martínez, G.; Olveira, G.; Valdés, S.; Soriguer, F.; Castaño, L.; Morcillo, S. Methylation levels of the SCD1 gene promoter and LINE-1 repeat region are associated with weight change: An intervention study. Mol. Nutr. Food Res. 2014, 58, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- De la Rocha, C.; Pérez-Mojica, J.E.; León, S.Z.; Cervantes-Paz, B.; Tristán-Flores, F.E.; Rodríguez-Ríos, D.; Molina-Torres, J.; Ramírez-Chávez, E.; Alvarado-Caudillo, Y.; Carmona, F.J.; et al. Associations between whole peripheral blood fatty acids and DNA methylation in humans. Sci. Rep. 2016, 6, 25867. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, C.F.; Nonino, C.B.; de Oliveira, B.A.; Pinhel, M.A.; Mansego, M.L.; Milagro, F.I.; Zulet, M.A.; Martinez, J.A. DNA methylation and hydroxymethylation levels in relation to two weight loss strategies: Energy-restricted diet or bariatric surgery. Obes. Surg. 2016, 26, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Trac, C.; Du, J.; Natarajan, R.; Schones, D.E. Persistent chromatin modifications induced by high fat diet. J. Biol. Chem. 2016, 291, 10446–10455. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.V.; Heintz-Buschart, A.; Ghosal, A.; Wampach, L.; Etheridge, A.; Galas, D.; Wilmes, P. Sources and functions of extracellular small RNAs in human circulation. Annu. Rev. Nutr. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Schober, A.; Nazari-Jahantigh, M.; Weber, C. MicroRNA-mediated mechanisms of the cellular stress response in atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Balasubramanian, S.; Rajasingh, S.; Patel, U.; Dhanasekaran, A.; Dawn, B.; Rajasingh, J. MicroRNA: A new therapeutic strategy for cardiovascular diseases. Trends Cardiovasc. Med. 2016, 26, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012, 110, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yang, C.; Han, Z. Circulating miR-499 as a potential biomarker for acute myocardial infarction. Ann. Transl. Med. 2016, 4, 135. [Google Scholar] [CrossRef] [PubMed]
- Párrizas, M.; Brugnara, L.; Esteban, Y.; González-Franquesa, A.; Canivell, S.; Murillo, S.; Gordillo-Bastidas, E.; Cussó, R.; Cadefau, J.A.; García-Roves, P.M.; et al. Circulating miR-192 and miR-193b are markers of prediabetes and are modulated by an exercise intervention. J. Clin. Endocrinol. Metab. 2015, 100, E407–E415. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Giardina, S.; Salas-Salvadó, J.; Arcelin, P.; Bulló, M. Chronic pistachio intake modulates circulating microRNAs related to glucose metabolism and insulin resistance in prediabetic subjects. Eur. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tabet, F.; Cuesta Torres, L.F.; Ong, K.L.; Shrestha, S.; Choteau, S.A.; Barter, P.J.; Clifton, P.; Rye, K.A. High-density lipoprotein-associated miR-223 is altered after diet-induced weight loss in overweight and obese males. PLoS ONE 2016, 11, e0151061. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Cardona-Alvarado, M.I.; Mercader, J.M.; Moreno-Navarrete, J.M.; Moreno, M.; Sabater, M.; Fuentes-Batllevell, N.; Ramírez-Chávez, E.; Ricart, W.; Molina-Torres, J.; et al. Circulating profiling reveals the effect of a polyunsaturated fatty acid-enriched diet on common microRNAs. J. Nutr. Biochem. 2015, 26, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Marques-Rocha, J.L.; Milagro, F.I.; Mansego, M.L.; Zulet, M.A.; Bressan, J.; Martínez, J.A. Expression of inflammation-related miRNAs in white blood cells from subjects with metabolic syndrome after 8 wk of following a Mediterranean diet-based weight loss program. Nutrition 2016, 32, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Nettleton, J.A.; Rotllan, N.; Tanaka, T.; Smith, C.E.; Lai, C.Q.; Parnell, L.D.; Lee, Y.C.; Lahti, J.; Lemaitre, R.N.; et al. Gain-of-function lipoprotein lipase variant rs13702 modulates lipid traits through disruption of a microRNA-410 seed site. Am. J. Hum. Genet. 2013, 92, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Han, D.Y.; Fraser, A.G.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn's disease patients. Hum. Genom. 2013, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, V.; Covas, M.I.; Muñoz-Aguayo, D.; Khymenets, O.; de la Torre, R.; Saez, G.; Tormos, M.C.; Toledo, E.; Marti, A.; Ruiz-Gutiérrez, V.; et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: A randomized controlled trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorente-Cortés, V.; Estruch, R.; Mena, M.P.; Ros, E.; González, M.A.; Fitó, M.; Lamuela-Raventós, R.M.; Badimon, L. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis 2010, 208, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Carraro, A.; Valente, R.; Iacopino, L.; Colica, C.; de Lorenzo, A. Intake of red wine in different meals modulates oxidized LDL level, oxidative and inflammatory gene expression in healthy people: A randomized crossover trial. Oxid. Med. Cell Longev. 2014, 2014, 681318. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Casillas, R.; Bulló, M.; Castañer, O.; Ros, E.; Sáez, G.T.; Toledo, E.; Estruch, R.; Ruiz-Gutiérrez, V.; Fitó, M.; et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur. J. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Castañer, O.; Konstantinidou, V.; Subirana, I.; Muñoz-Aguayo, D.; Blanchart, G.; Gaixas, S.; de la Torre, R.; Farré, M.; Sáez, G.T.; et al. Effect of olive oil phenolic compounds on the expression of blood pressure-related genes in healthy individuals. Eur. J. Nutr. 2015. [Google Scholar]
- Konstantinidou, V.; Covas, M.I.; Sola, R.; Fitó, M. Up-to date knowledge on the in vivo transcriptomic effect of the Mediterranean diet in humans. Mol. Nutr. Food Res. 2013, 57, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M.; Konstantinidou, V. Nutritional genomics and the Mediterranean diet's effects on human cardiovascular health. Nutrients 2016, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Newgard, C.B. Integrated metabolomics and genomics: Systems approaches to biomarkers and mechanisms of cardiovascular disease. Circ. Cardiovasc. Genet. 2015, 8, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aloy, M.; Llorach, R.; Urpi-Sarda, M.; Tulipani, S.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Fitó, M.; Ros, E.; Salas-Salvadó, J.; et al. Novel multimetabolite prediction of walnut consumption by a urinary biomarker model in a free-living population: The PREDIMED study. J. Proteome Res. 2014, 13, 3476–3483. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aloy, M.; Llorach, R.; Urpi-Sarda, M.; Jáuregui, O.; Corella, D.; Ruiz-Canela, M.; Salas-Salvadó, J.; Fitó, M.; Ros, E.; Estruch, R.; et al. A metabolomics-driven approach to predict cocoa product consumption by designing a multimetabolite biomarker model in free-living subjects from the PREDIMED study. Mol. Nutr. Food Res. 2015, 59, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Martinez, J.A.; de la Iglesia, R.; Lopez-Legarrea, P.; Poutanen, K.; Hanhineva, K.; Zulet, M.L. Effects of short- and long-term Mediterranean-based dietary treatment on plasma LC-QTOF/MS metabolic profiling of subjects with metabolic syndrome features: The metabolic syndrome reduction in navarra (RESMENA) randomized controlled trial. Mol. Nutr. Food Res. 2015, 59, 711–728. [Google Scholar] [CrossRef] [PubMed]
- González-Guardia, L.; Yubero-Serrano, E.M.; Delgado-Lista, J.; Perez-Martinez, P.; Garcia-Rios, A.; Marin, C.; Camargo, A.; Delgado-Casado, N.; Roche, H.M.; Perez-Jimenez, F.; et al. Effects of the Mediterranean diet supplemented with coenzyme q10 on metabolomic profiles in elderly men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, H.; Brennan, L. Metabolomics as a tool in the identification of dietary biomarkers. Proc. Nutr. Soc. 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dona, A.C.; Coffey, S.; Figtree, G. Translational and emerging clinical applications of metabolomics in cardiovascular disease diagnosis and treatment. Eur. J. Prev. Cardiol. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Zheng, Y.; Ruiz-Canela, M.; Hruby, A.; Martínez-González, M.A.; Clish, C.B.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; et al. Plasma acylcarnitines and risk of cardiovascular disease: Effect of Mediterranean diet interventions. Am. J. Clin. Nutr. 2016, 103, 1408–1416. [Google Scholar]
- Ruiz-Canela, M.; Toledo, E.; Clish, C.B.; Hruby, A.; Liang, L.; Salas-Salvadó, J.; Razquin, C.; Corella, D.; Estruch, R.; Ros, E.; et al. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin. Chem. 2016, 62, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Hyötyläinen, T.; Bondia-Pons, I.; Orešič, M. Lipidomics in nutrition and food research. Mol. Nutr. Food Res. 2013, 57, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.F.; Allayee, H.; Gerszten, R.E.; Ideraabdullah, F.; Kris-Etherton, P.M.; Ordovás, J.M.; Rimm, E.B.; Wang, T.J.; Bennett, B.J. Nutrigenomics, the microbiome, and gene-environment interactions: New directions in cardiovascular disease research, prevention, and treatment: A scientific statement from the american heart association. Circ. Cardiovasc. Genet. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Huffman, K.M.; Redman, L.M.; Landerman, L.R.; Pieper, C.F.; Stevens, R.D.; Muehlbauer, M.J.; Wenner, B.R.; Bain, J.R.; Kraus, V.B.; Newgard, C.B.; et al. Caloric restriction alters the metabolic response to a mixed-meal: Results from a randomized, controlled trial. PLoS ONE 2012, 7, e28190. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Barlow, C.K.; Mellett, N.A.; Mundra, P.A.; Bonham, M.P.; Larsen, A.; Cameron-Smith, D.; Sinclair, A.; Nestel, P.J.; Wong, G. Postprandial plasma phospholipids in men are influenced by the source of dietary fat. J. Nutr. 2015, 145, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, M.; Schwab, U.; Erkkilä, A.; Seppänen-Laakso, T.; Hannila, M.L.; Mussalo, H.; Lehto, S.; Uusitupa, M.; Gylling, H.; Oresic, M. Fatty fish intake decreases lipids related to inflammation and insulin signaling—A lipidomics approach. PLoS ONE 2009, 4, e5258. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Pöhö, P.; Bozzetto, L.; Vetrani, C.; Patti, L.; Aura, A.M.; Annuzzi, G.; Hyötyläinen, T.; Rivellese, A.A.; Orešič, M. Isoenergetic diets differing in their n-3 fatty acid and polyphenol content reflect different plasma and HDL-fraction lipidomic profiles in subjects at high cardiovascular risk. Mol. Nutr. Food Res. 2014, 58, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, M.; Schwab, U.; Kolehmainen, M.; Paananen, J.; Nygren, H.; Seppänen-Laakso, T.; Poutanen, K.; Hyötyläinen, T.; Risérus, U.; Brader, L.; et al. A healthy nordic diet alters the plasma lipidomic profile in adults with features of metabolic syndrome in a multicenter randomized dietary intervention. J. Nutr. 2016, 146, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G.; Padro, T. Systems biology approaches to understand the effects of nutrition and promote health. Br. J. Clin. Pharmacol. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.G.; Wu, Y.; Jha, P.; Dubuis, S.; Blattmann, P.; Argmann, C.A.; Houten, S.M.; Amariuta, T.; Wolski, W.; Zamboni, N.; et al. Systems proteomics of liver mitochondria function. Science 2016, 352, aad0189. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, A.; Kloosterman, B.; Visser, R.G.; Maliepaard, C. Integration of multi-omics data for prediction of phenotypic traits using random forest. BMC Bioinform. 2016, 17, 180. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, H.S.; Kim, Y.K.; Park, S.; Kim, J.M.; Yun, J.H.; Yu, H.Y.; Kim, B.J. Association of metabolites with obesity and type 2 diabetes based on FTO genotype. PLoS ONE 2016, 11, e0156612. [Google Scholar] [CrossRef] [PubMed]
- Hartiala, J.A.; Tang, W.H.; Wang, Z.; Crow, A.L.; Stewart, A.F.; Roberts, R.; McPherson, R.; Erdmann, J.; Willenborg, C.; Hazen, S.L.; et al. Genome-wide association study and targeted metabolomics identifies sex-specific association of CPS1 with coronary artery disease. Nat. Commun. 2016, 7, 10558. [Google Scholar] [CrossRef] [PubMed]
- Gieger, C.; Geistlinger, L.; Altmaier, E.; Hrabé de Angelis, M.; Kronenberg, F.; Meitinger, T.; Mewes, H.W.; Wichmann, H.E.; Weinberger, K.M.; Adamski, J.; et al. Genetics meets metabolomics: A genome-wide association study of metabolite profiles in human serum. PLoS Genet. 2008, 4, e1000282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffler, J.; Friedrich, N.; Arnold, M.; Kacprowski, T.; Rueedi, R.; Altmaier, E.; Bergmann, S.; Budde, K.; Gieger, C.; Homuth, G.; et al. Genome-wide association study with targeted and non-targeted nmr metabolomics identifies 15 novel loci of urinary human metabolic individuality. PLoS Genet. 2015, 11, e1005487. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.K.; Zeilinger, S.; Kastenmüller, G.; Römisch-Margl, W.; Brugger, M.; Peters, A.; Meisinger, C.; Strauch, K.; Hengstenberg, C.; Pagel, P.; et al. Epigenetics meets metabolomics: An epigenome-wide association study with blood serum metabolic traits. Hum. Mol. Genet. 2014, 23, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.V.; Hu, Y.J. Integrative Analysis of multi-omics data for discovery and functional studies of complex human diseases. Adv. Genet. 2016, 93, 147–190. [Google Scholar] [PubMed]
- Vivek-Ananth, R.P.; Samal, A. Advances in the integration of transcriptional regulatory information into genome-scale metabolic models. Biosystems 2016, in press. [Google Scholar] [CrossRef] [PubMed]
| Underlying Mechanisms | References |
|---|---|
| 1. Richness in antioxidants | |
| 1.1. Protects blood and tissue components from oxidative stress | [42] |
| 1.2. Limits the oxidation of unsaturated fatty acids during intestinal transit | [42] |
| 2. MUFA 1 and PUFA 2 content in membranes preserves membrane fluidity and functionality | [43] |
| 3. Richness in nitrates | |
| 3.1. Production of nitrolipids by the nitration of unsaturated fatty acids | [44] |
| 3.2. Nitric oxide generation from the nitrate-nitrite-nitric oxide pathway | [37] |
| 4. Modulation of microbial populations and activities | [45,46] |
| 5. Temporal distribution of food consumption throughout the day | [47] |
| 6. Synergistic interactions and cumulative effects betwee different foods and nutrients | [42] |
| 7. Modulation of gene expression | [48] |
| 8. Modulation of metabolite production | [48] |
| Omic-Based Biomarkers | Description | References |
|---|---|---|
| Genetic biomarkers | Based on changes in DNA, single nucleotide polymorphisms (SNP). Examples: | |
| SNPs in the lactase gene (LCT) as proxies of milk consumption in Mendelian randomization analyses. | [52] | |
| SNPs in the lipoprotein lipase (LPL) gene as biomarkers of genetic risk of stroke. | [40] | |
| Epigenetic biomarkers | Biomarkers based on the main epigenetic regulators: DNA methylation, histone modification, and non-coding RNAs. Examples: | |
| DNA hypermetylation or hypomethylation of specific genes depending on food intake; Levels of circulating microRNAs associated with several nutrition-related diseases. | [53,54] | |
| Transcriptomic biomarkers | Biomarkers based on RNA expression (whole transcriptome or differences in the expression of selected genes). Example: | [55] |
| Differences in the gene expression profile in subjects following a Mediterranean diet in comparison with control subjects. | [56] | |
| Proteomic biomarkers | Biomarkers based on the study of the proteome. | [57] |
| Lipidomic biomarkers | Biomarkers based on the study of the lipidome (comprehensive analysis of the molecular lipid species). | [58] |
| Metabolomic biomarkers | Biomarkers based on the study of the metabolome [the entire small molecule (metabolite) component of a system]. Metabolites (including peptides, lipids, nucleotides, carbohydrates, amino acids, and many other classes of small molecules) are generally defined as having an atomic mass of less than 1.5 kDa and can be exogenous, endogenous, or derived from the microbiome. Example: | [59] |
| The 1H NMR urinary profile in subjects following a traditional Mediterranean diet in comparison with the urinary profile of subject on a low fat diet. | [60] |
| Reference | Population Analyzed | Phenotype Analyzed | Study Characteristics | Main Results |
|---|---|---|---|---|
| Corella et al., 2013 [63] | 7018 high cardiovascular risk subjects participating in the PREDIMED study | Stroke incidence | Randomized controlled trial with MedDiet (two groups pooled) versus a control diet (4.8 years of median follow-up) | The association between the TCF7L2-rs7903146 (C>T) polymorphism and stroke was modulated by the intervention with MedDiet. TT subjects had a higher stroke incidence in the control group (p = 0.006 compared with CC), whereas dietary intervention with MedDiet reduced stroke incidence in TT homozygotes (p = 0.892 compared with CC). |
| Ortega Azorín et al., 2014 [64] | 7166 high cardiovascular risk subjects participating in the PREDIMED study | Myocardial infarction incidence | Randomized controlled trial with MedDiet (two groups pooled) versus a control diet (4.8 years of median follow-up) | The association between the rs3812316 C>G SNP and myocardial infarction incidence was modulated by the intervention with MedDiet. Carriers of the G allele had significantly lower incidence of myocardial infarction only in the MedDiet intervention group. |
| Gómez-Delgado et al., 2014 [75] | 507 metabolic syndrome (MetS) patients selected from the CORDIOPREV clinical trial | Triglycerides and high sensitivity C-reactive protein (hsCRP) | Randomized trial: MedDiet, compared with a low-fat diet (1 year of follow-up) | The rs1800629 polymorphism at the TNFA gene interacted with intervention with MedDiet to influence triglyceride metabolism and inflammation status in MetS subjects. The decrease in triglycerides and hsCRP was statistically significant in G/G subjects compared with carriers of the minor A-allele. |
| Di Daniele et al., 2014 [76] | 40 male patients with chronic kidney disease | Homocysteine levels and other biochemical parameters | Dietary intervention with an Italian Mediterranean organic diet (IMOD) versus low-protein diet (LPD) for 14 days | They found a significant interaction between MTHFR C667T polymorphism and the IMOD on homocysteine levels compared to LPD The IMOD resulted in significant improvement of homocysteine levels in TT. |
| Corella et al., 2014 [77] | 7187 high cardiovascular risk subjects participating in the PREDIMED study | Fasting triglycerides and stroke incidence | Randomized controlled trial with MedDiet (two groups pooled) versus a control diet (4.8 years of median follow-up) | The rs13702 T>C in the 3′ untranslated region of the LPL gene interacted with the intervention with MedDiet in determining changes in triglycerides and stroke incidence. The decreasing effect of the C allele on triglycerides and stroke incidence was only significant in the MedDiet intervention group. |
| Corella et al., 2016 [78] | 3671 non-diabetic subjects participating in the PREDIMED study | Type-2 diabetes incidence | Randomized controlled trial with MedDiet (two groups pooled) versus a control diet (4.8 years of median follow-up) | The CLOCK-rs4580704 C>G SNP was associated with incidence of type-2 diabetes, with variant allele (G) carriers showing decreased incidence (dominant model) compared with CC homozygotes. However, this protection was more significant in the MedDiet intervention group (p < 0.001) than in the control group (p = 0.818). |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitó, M.; Melander, O.; Martínez, J.A.; Toledo, E.; Carpéné, C.; Corella, D. Advances in Integrating Traditional and Omic Biomarkers When Analyzing the Effects of the Mediterranean Diet Intervention in Cardiovascular Prevention. Int. J. Mol. Sci. 2016, 17, 1469. https://doi.org/10.3390/ijms17091469
Fitó M, Melander O, Martínez JA, Toledo E, Carpéné C, Corella D. Advances in Integrating Traditional and Omic Biomarkers When Analyzing the Effects of the Mediterranean Diet Intervention in Cardiovascular Prevention. International Journal of Molecular Sciences. 2016; 17(9):1469. https://doi.org/10.3390/ijms17091469
Chicago/Turabian StyleFitó, Montserrat, Olle Melander, José Alfredo Martínez, Estefanía Toledo, Christian Carpéné, and Dolores Corella. 2016. "Advances in Integrating Traditional and Omic Biomarkers When Analyzing the Effects of the Mediterranean Diet Intervention in Cardiovascular Prevention" International Journal of Molecular Sciences 17, no. 9: 1469. https://doi.org/10.3390/ijms17091469
APA StyleFitó, M., Melander, O., Martínez, J. A., Toledo, E., Carpéné, C., & Corella, D. (2016). Advances in Integrating Traditional and Omic Biomarkers When Analyzing the Effects of the Mediterranean Diet Intervention in Cardiovascular Prevention. International Journal of Molecular Sciences, 17(9), 1469. https://doi.org/10.3390/ijms17091469