Regulation of Translocator Protein 18 kDa (TSPO) Expression in Rat and Human Male Germ Cells
Abstract
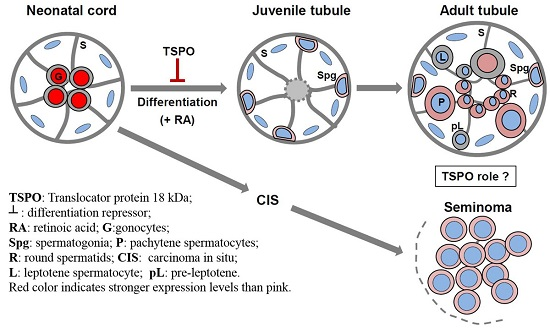
:1. Introduction
2. Results
2.1. Changes in TSPO Expression during Gonocyte Differentiation and in Spermatogonia
2.2. TSPO Expression in Differentiating F9 Mouse Embryonal Carcinoma Cells
2.3. Effects of TSPO Knockdown on Gonocyte Differentiation and on the Expression of Germ Cell Specific Genes
2.4. TSPO Expression in Normal Human Adult Testicular Tissues
2.5. TSPO Expression in Seminomas
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Germ Cell Isolation
4.3. Gonocyte Treatment
4.4. TSPO Knockdown Using Silencing RNA
4.5. F9 Mouse Embryonal Carcinoma Cell Culture
4.6. Human Specimens
4.7. RNA Extraction and cDNA Synthesis
4.8. Quantitative Real Time PCR (qPCR)
4.9. Immunoblot Analysis
4.10. Immunocytochemistry (ICC)
4.11. Immunofluorescence (IF)
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Delavoie, F.; Li, H.; Hardwick, M.; Robert, J.C.; Giatzakis, C.; Peranzi, G.; Yao, Z.X.; Maccario, J.; Lacapere, J.J.; Papadopoulos, V. In vivo and in vitro peripheral-type benzodiazepine receptor polymerization: Functional significance in drug ligand and cholesterol binding. Biochemistry 2003, 42, 4506–4519. [Google Scholar] [CrossRef] [PubMed]
- Midzak, A.; Rone, M.; Aghazadeh, Y.; Culty, M.; Papadopoulos, V. Mitochondrial protein import and the genesis of steroidogenic mitochondria. Mol. Cell. Endocrinol. 2011, 336, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, M.; Fertikh, D.; Culty, M.; Li, H.; Vidic, B.; Papadopoulos, V. Peripheral-type benzodiazepine receptor (TSPO) in human breast cancer: Correlation of breast cancer cell aggressive phenotype with TSPO expression, nuclear localization, and TSPO-mediated cell proliferation and nuclear transport of cholesterol. Cancer Res. 1999, 59, 831–842. [Google Scholar] [PubMed]
- Corsi, L.; Geminiani, E.; Avallone, R.; Baraldi, M. Nuclear location-dependent role of peripheral benzodiazepine receptor (PBR) in hepatic tumoral cell lines proliferation. Life Sci. 2005, 76, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Papadopoulos, V.; Gavish, M. Channel-like functions of the 18 kDa Translocator protein (TSPO): Regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr. Pharm. Des. 2007, 13, 2385–2405. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, A.; Papadopoulos, V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol. Cell. Endocrinol. 2010, 327, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Manku, G.; Wang, Y.; Thuillier, R.; Rhodes, C.; Culty, M. Developmental expression of the Translocator protein 18 kDa (TSPO) in testicular germ cells. Curr. Mol. Med. 2012, 12, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Culty, M.; Liu, Y.; Manku, G.; Chan, W.Y.; Papadopoulos, V. Expression of steroidogenesis-related genes in murine male germ cells. Steroids 2012, 103, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Culty, M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res. C Embryo Today 2009, 876, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Culty, M. Gonocytes, from the fifties to the present: Is there a reason to change the name? Biol. Reprod. 2013, 89, 46. [Google Scholar] [CrossRef] [PubMed]
- Manku, G.; Culty, M. Mammalian gonocyte and spermatogonia differentiation: Recent advances and remaining challenges. Reproduction 2015, 149, R139–R157. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Papadopoulos, V.; Vidic, B.; Dym, M.; Culty, M. Regulation of rat testis gonocyte proliferation by platelet-derived growth factor and estradiol: Identification of signalling mechanisms involved. Endocrinology 1997, 138, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, R.; Mazer, M.; Manku, G.; Boisvert, A.; Wang, Y.; Culty, M. Interdependence of PDGF and estrogen signaling pathways in inducing neonatal rat testicular gonocytes proliferation. Biol. Reprod. 2010, 82, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Culty, M. Identification and distribution of a novel platelet-derived growth factor receptor beta variant: Effect of retinoic acid and involvement in cell differentiation. Endocrinology 2007, 148, 2233–2250. [Google Scholar] [CrossRef] [PubMed]
- Manku, G.; Wang, Y.; Merkbaoui, V.; Boisvert, A.; Ye, X.; Blonder, J.; Culty, M. Role of Retinoic Acid and Platelet-Derived Growth Factor Receptor crosstalk in the regulation of neonatal gonocyte and embryonal carcinoma cell differentiation. Endocrinology 2015, 1, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Manku, G.; Culty, M. Dynamic changes in the expression of apoptosis-related genes in differentiating gonocytes and in seminomas. Asian J. Androl. 2015, 7, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.E.; Berthelsen, J.G.; Giwercman, A.; Muller, J. Carcinoma-in-situ of the testis: Possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int. J. Androl. 1987, 10, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, E.; Matsuda, T.; Thonneau, P. Increasing incidence of testicular cancer worldwide: A review. J. Urol. 2003, 170, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Manku, G.; Wing, S.S.; Culty, M. Expression of the Ubiquitin Proteasome System in Neonatal Rat Gonocytes and Spermatogonia: Role in Gonocyte Differentiation. Biol. Reprod. 2012, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Unhavaithaya, Y.; Hao, Y.; Beyret, E.; Yin, H.; Kuramochi-Miyagawa, S.; Nakano, T.; Lin, H. MILI, a PIWI-interacting RNA-binding protein, is required for germ line stem cell self-renewal and appears to positively regulate translation. J. Biol. Chem. 2009, 284, 6507–6519. [Google Scholar] [CrossRef] [PubMed]
- Carmell, M.A.; Girard, A.; van de Kant, H.J.; Bourc’his, D.; Bestor, T.H.; de Rooij, D.G.; Hannon, G.J. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell 2007, 12, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Kallajoki, M.; Virtanen, I.; Suominen, J. The fate of acrosomal staining during the acrosome reaction of human spermatozoa as revealed by a monoclonal antibody and PNA-lectin. Int. J. Androl. 1986, 9, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Looijenga, L.H.; Stoop, H.; Biermann, K. Testicular cancer: Biology and biomarkers. Virchows Arch. 2014, 464, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Pauls, K.; Jager, R.; Weber, S.; Wardelmann, E.; Koch, A.; Buttner, R.; Schorle, H. Transcription factor AP-2gamma, a novel marker of gonocytes and seminomatous germ cell tumors. Int. J. Cancer 2005, 115, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V. On the role of the translocator protein (18-kDa) TSPO in steroid hormone biosynthesis. Endocrinology 2014, 155, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Campioli, E.; Midzak, A.; Culty, M.; Papadopoulos, V. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc. Natl. Acad. Sci. USA 2015, 112, 7261–7266. [Google Scholar] [CrossRef] [PubMed]
- Goertz, M.J.; Wu, Z.; Gallardo, T.D.; Hamra, F.K.; Castrillon, D.H. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J. Clin. Investig. 2011, 121, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Rochette-Egly, C.; Chambon, P. F9 embryo carcinoma cells: A cell autonomous model to study the functional selectivity of RARs and RXRs in retinoid signaling. Histol. Histopathol. 2001, 16, 909–922. [Google Scholar] [PubMed]
- Kwon, J.T.; Jin, S.; Choi, H.; Kim, J.; Jeong, J.; Kim, J.; Kim, Y.; Cho, B.N.; Cho, C. Identification and characterization of germ cell genes expressed in the F9 testicular teratoma stem cell line. PLoS ONE 2014, 9, e103837. [Google Scholar] [CrossRef] [PubMed]
- Campioli, E.; Batarseh, A.; Li, J.; Papadopoulos, V. The endocrine disruptor mono-(2-ethylhexyl) phthalate affects the differentiation of human liposarcoma cells (SW 872). PLoS ONE 2011, 6, e28750. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Papadopoulos, V. Translocator protein (18 kDa) as a pharmacological target in adipocytes to regulate glucose homeostasis. Biochem. Pharmacol. 2015, 97, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Barrios, F.; Filipponi, D.; Pellegrini, M.; Paronetto, M.P.; Di Siena, S.; Geremia, R.; Rossi, P.; de Felici, M.; Jannini, E.A.; Dolci, S. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J. Cell Sci. 2010, 123, 871–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.C.; Lin, H. Beyond transposons: The epigenetic and somatic functions of the Piwi-piRNA mechanism. Curr. Opin. Cell Biol. 2013, 25, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Lindemann, P.; Feuilloley, M.G.; Papadopoulos, V. Structural and functional evolution of the translocator protein (18 kDa). Curr. Mol. Med. 2012, 12, 369–386. [Google Scholar] [CrossRef] [PubMed]
- O’Beirne, G.B.; Woods, M.J.; Williams, D.C. Two subcellular locations for peripheral-type benzodiazepine acceptors in rat liver. Eur. J. Biochem. 1990, 188, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Janczar, K.; Su, Z.; Raccagni, I.; Anfosso, A.; Kelly, C.; Durrenberger, P.F.; Gerhard, A.; Roncaroli, F. The 18 kDa mitochondrial translocator protein in gliomas: From the bench to bedside. Biochem. Soc. Trans. 2015, 43, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Gavish, M.; Cohen, S.; Nagler, R. Cigarette smoke effects on TSPO and VDAC expression in a cellular lung cancer model. Eur. J. Cancer Prev. 2016, 25, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gallo, K.A. The 18 kDa translocator protein (TSPO) disrupts mammary epithelial morphogenesis and promotes breast cancer cell migration. PLoS ONE 2013, 8, e71258. [Google Scholar] [CrossRef] [PubMed]
- Galiegue, S.; Casellas, P.; Kramar, A.; Tinel, N.; Simony-Lafontaine, J. Immunohistochemical assessment of the peripheral benzodiazepine receptor in breast cancer and its relationship with survival. Clin. Cancer Res. 2004, 10, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Slack, R.S.; Li, W.; Papadopoulos, V. Expression of peripheral benzodiazepine receptor (PBR) in human tumors: Relationship to breast, colorectal, and prostate tumor progression. J. Recept. Signal Transduct. Res. 2003, 23, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, A.; Barlow, K.D.; Martinez-Arguelles, D.B.; Papadopoulos, V. Functional characterization of the human translocator protein (18 kDa) gene promoter in human breast cancer cell lines. Biochim. Biophys. Acta 2012, 1819, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Manku, G.; Mazer, M.; Culty, M. Neonatal testicular gonocytes isolation and processing for immunocytochemical analysis. Methods Mol. Biol. 2012, 825, 17–29. [Google Scholar] [PubMed]
- Manku, G.; Hueso, A.; Brimo, F.; Chan, P.; Gonzalez-Peramato, P.; Jabado, N.; Riazalhosseini, Y.; Gayden, T.; Bourgey, M.; Culty, M. Changes in claudin expression profiles during gonocyte differentiation and high expression in seminomas. Andrology 2016, 4, 95–110. [Google Scholar] [CrossRef] [PubMed]





| Patient | Pathology | Age | Tissue Type |
|---|---|---|---|
| Samples for qPCR analysis | |||
| 1 | Normal | Normal spermatogenesis, Vasectomy patient | |
| 2 | Normal | Normal spermatogenesis, Vasectomy patient | |
| 3 | Normal | Normal spermatogenesis, Vasectomy patient | |
| 4 | Seminoma | Left testis, Seminoma, Age 36, Stage I | |
| 5 | Seminoma | Right testis, Seminoma, Age 26, Stage I | |
| 6 | Seminoma | Right testis, Seminoma, Age 50, Stage I | |
| Samples for IF analysis | |||
| 1 | Normal | 73 | Normal testicular parenchyma |
| 2 | Normal | 57 | Normal testicular parenchyma |
| 3 | Normal | 73 | Normal testicular parenchyma |
| 4 | Seminoma | NA | 100% Seminoma |
| 5 | Seminoma | NA | 100% Seminoma |
| 6 | Seminoma | 38 | 100% Seminoma |
| 7 | Seminoma | NA | 100% Seminoma |
| # | Sense Strand | Antisense Strand |
|---|---|---|
| 1 | 5′-CCUACAUAAUCUGGAAAGAGCUGGG-3 | 5′-CCCAGCUCUUUCCAGAUUAUGUAGGAG-3′ |
| 2 | 5′-GCAGUUGCAAUCACUAUGUCUCAAT-3′ | 5′-AUUGAGACAUAGUGAUUGCAACUGCUG-3′ |
| 3 | 5′-CACCUAGCCAUCAGGAAUGCAGCCC-3′ | 5′-AUUGAGACAUAGUGAUUGCAACUGCUG-3′ |
| Species | Gene | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| Rat | Tspo | cgcaATGGGAGCCTACTTTGTGcG | GCCAGGAGGGTTTCTGCAAG |
| Rat | Stra8 | TGCTTTTGATGTGGCGAGCT | GCGCTGATGTTAGACAGACGCT |
| Rat | Mili | cggaaGACATCCAGTACAGAGTCATTCcG | GGGTCTCTTCTTGCTCGCTGA |
| Rat | Miwi2 | AGCTGCACAGGTATTCCGCTAGAA | ATGGCAGACAGGACTTCGTCGATT |
| Rat | 18s | cgggTGCTCTTAGCTGAGTGTCCcG | CTCGGGCCTGCTTTGAACAC |
| Mouse | Tspo | CCCGCTTGCTGTACCCTTACC | CACCGCATACATAGTAGTTGAGCAC |
| Mouse | 18s | CGGAATCTTAATCATGGCCTCAGTTC | ACCGCAGCTAGGAATAATGGAAT |
| Human | TSPO | GGTGGATCTCCTGCTGGTCA | CGACCAATACGCAGTAGTTGAGTGTG |
| Human | 18S | cggacTGTGATGCCCTTAGATGTCcG | GTAGGGTAGGCACACGCTGAG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manku, G.; Culty, M. Regulation of Translocator Protein 18 kDa (TSPO) Expression in Rat and Human Male Germ Cells. Int. J. Mol. Sci. 2016, 17, 1486. https://doi.org/10.3390/ijms17091486
Manku G, Culty M. Regulation of Translocator Protein 18 kDa (TSPO) Expression in Rat and Human Male Germ Cells. International Journal of Molecular Sciences. 2016; 17(9):1486. https://doi.org/10.3390/ijms17091486
Chicago/Turabian StyleManku, Gurpreet, and Martine Culty. 2016. "Regulation of Translocator Protein 18 kDa (TSPO) Expression in Rat and Human Male Germ Cells" International Journal of Molecular Sciences 17, no. 9: 1486. https://doi.org/10.3390/ijms17091486
APA StyleManku, G., & Culty, M. (2016). Regulation of Translocator Protein 18 kDa (TSPO) Expression in Rat and Human Male Germ Cells. International Journal of Molecular Sciences, 17(9), 1486. https://doi.org/10.3390/ijms17091486